

Elexes Team
Elexes medical consulting is one of the leading regulatory & compliance consultant for several industries: Medical device, Pharmaceuticals, Cosmetics, Food, and Biologics.

Diagnosis plays a vital role in curing a disease. If the diagnosis itself is wrong, the disease cannot be treated. Osteosarcoma, a dangerous but common form of bone cancer which mostly affects the population of children and young adults (15 – 24), is often being misdiagnosed as growing pains or muscle strains, according to the Bone Cancer Research Trust (BCRT). Due to improved awareness about the importance of diagnosis, Clinical Laboratories been gaining more interest now than ever. However, Clinical Laboratories in the States were reported with the wrong diagnosis and poor quality of services, prior to 1988.
The US Congress conducted several investigations of testing performed in the Physician Office Laboratories (POLs) and other labs. One major initiate was –The Clinical Laboratory Improvement Amendments (CLIA) which was passed by the Congress in 1988 to set standards designed for improving the quality in all laboratory testing which also includes specifications for quality control and assurance, patient test management, personnel and proficiency testing.
Approximately 260,000 laboratory entities are covered under CLIA. Labs and POLs operating in New York or Washington are exempt from CLIA. The CLIA database contains information about commercially marketed in-vitro systems and tests. The database is updated weekly and has been maintained by the FDA since January 31, 2000. Prior to 2000, the CDC (Center for Diseases Control and Prevention) used to maintain the database.
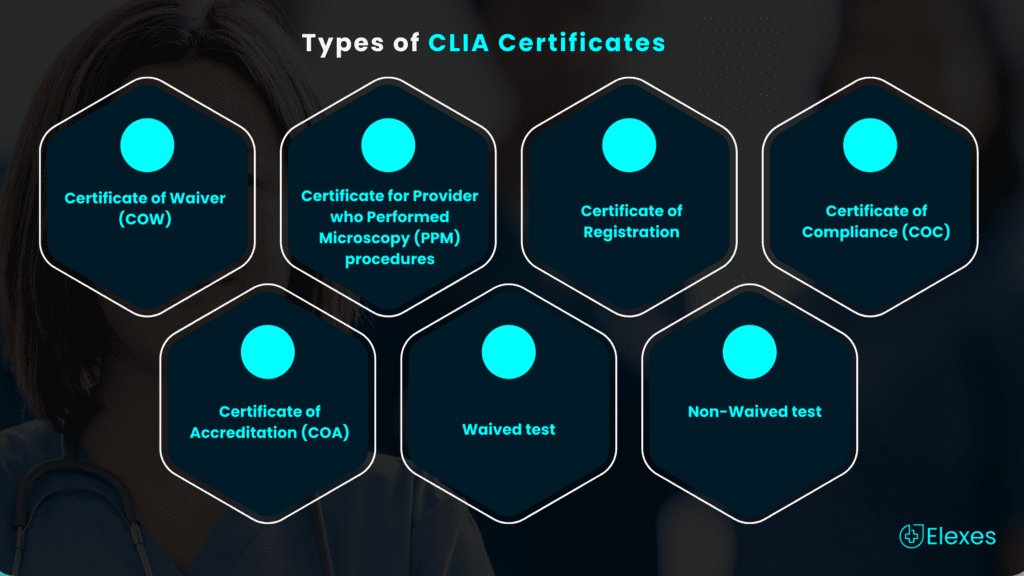
◉ Certificate of Waiver (COW) – Issued for performing only waived tests.
◉ Certificate for Provider who Performed Microscopy (PPM) procedures –Issued when a microscopy procedure (among a list of procedures included under this certificate) is to be performed on a patient.
◉ Certificate of Registration – Issued to the laboratory to conduct nonwaived testing until the laboratory is inspected to determine its compliance with the CLIA regulations.
◉ Certificate of Compliance (COC) – It is issued once the State Department of Health conducts the inspection and determines that the laboratory is compliant with all applicable CLIA requirements.
◉ Certificate of Accreditation (COA) – It is issued on the basis of the laboratory’s competence assessment by an accreditation organization approved by CMS.
NOTE: All types of certificates are valid for 2 years.
![]()
![]() ➜ Waived test – Simple laboratory examinations and procedures that have an insignificant risk of an erroneous result. Few of the waived tests include: Blood Glucose monitoring, Platelet Count, and Urine qualitative dipstick analysis.
➜ Waived test – Simple laboratory examinations and procedures that have an insignificant risk of an erroneous result. Few of the waived tests include: Blood Glucose monitoring, Platelet Count, and Urine qualitative dipstick analysis.
➜ Non-Waived test – It is a moderate and/or high complexity testing. Non-waived tests include: Hematology and Toxicology
A non-waived is further categorized into PPM and Moderate/High Complexity. PPM tests are different from moderate or high complexity tests. A test or device is classified as moderate or high, based on 7 criteria. The decision for classification of device or test is based on a ‘scorecard’. Each criterion can be marked as 1, 2 or 3 based on the level of complexity (3 being the highest and 1 being least complex). All the scores are then added to get an aggregate score. If the total score is 12 or less, it is considered as a moderate test. If the total score is greater than 12 it is considered as high complexity. The 7 criteria for classification are as follows:
To get a CLIA certification the Sponsor needs to fill CMS116 form and submit it to the respective State Agency. You can also contact professional service firms like Elexes Medical Consulting to assist in submitting for assisting in the certification process.
Elexes medical consulting is one of the leading regulatory & compliance consultant for several industries: Medical device, Pharmaceuticals, Cosmetics, Food, and Biologics.

