




When it comes to placing medical devices on the market in the European Union, the Medical Devices Regulation has taken the role of the Medical Device Directive (93/42/EEC).
All prior notices about the EU MDR transition pointed to the 26th of May, 2024 as the last day of the MDR transition phase. However, recently there have been some updates.
The European Commission proposed an extended transition time on January 6, 2023, based on the risk classification of the medical device.
The standards for safety and performance set out in the EU MDR will not be altered by this extension. Manufacturers are essentially given extra time to adapt their processes to the regulation’s new requirements.
⦿ To prevent the shortage of devices in the European countries
⦿ To provide more time for the manufacturers to get certified by the Notified Bodies (NBs) under new applicable regulations
⦿ To allow more NBs to qualify to be eligible for conducting an MDR review
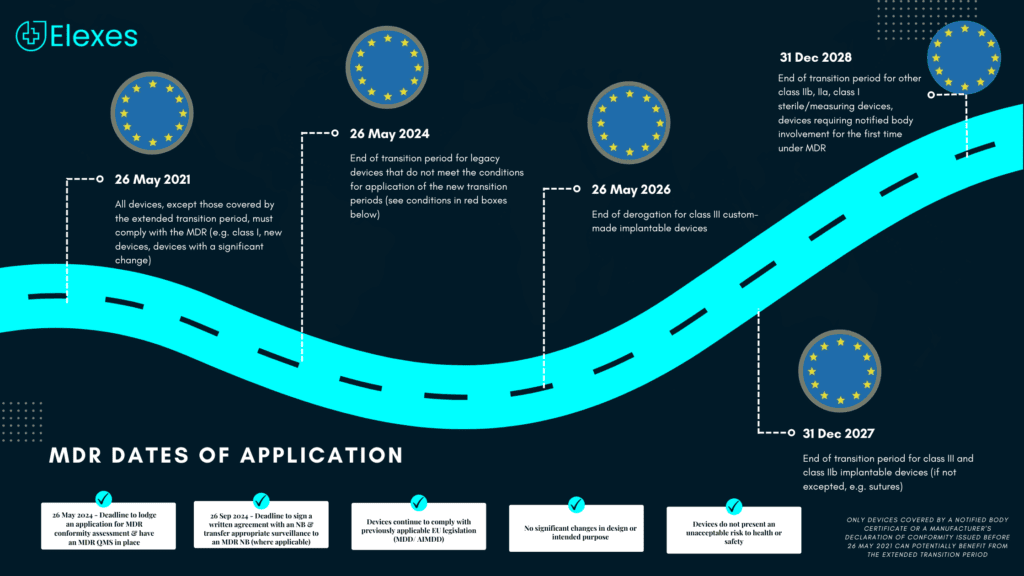

⦿ From May 26, 2024 to Dec 31, 2027 for Class III, Class IIB implantable devices
⦿ From May 26, 2024 to Dec 31, 2028 for Class IIB non-implantable, Class IIA, and Class I devices
This applies to medical devices covered by a certificate or a Declaration of Conformity (DoC) issued before May 26, 2021.
Under this proposal, manufacturers of Class III implantable custom-made devices would have until May 26, 2026, to get certified by an NB.
Certificates issued before the MDR’s implementation in the European Union on May 26, 2021, would have their expiration dates extended under the new proposal.
The Commission also intends to eliminate the “sell-off” deadline imposed under MDR and IVDR that mandates the needless disposal of some safe medical equipment.
Elexes medical consulting is one of the leading regulatory & compliance consultant for several industries: Medical device, Pharmaceuticals, Cosmetics, Food, and Biologics.



