
Compliance Assurance
Stay compliant with industry regulations and standards.
Cost-effective
Achieve regulatory success with Elexes, all within your budget.

PC: Canva
Canada boasts one of the largest and most regulated markets for healthcare. Whether introducing a new medical device or making modifications to existing ones, the trick becomes navigating through the very complex regulatory pathway of Health Canada.
At Elexes, our experienced Health Canada consultants help you streamline your licensing and documentation processes while ensuring full compliance with Canadian Medical Device Regulations (CMDR).
Canada’s licensing process may resemble other jurisdictions, but it involves unique requirements that require a deep understanding of both the CMDR and Canadian market expectations.
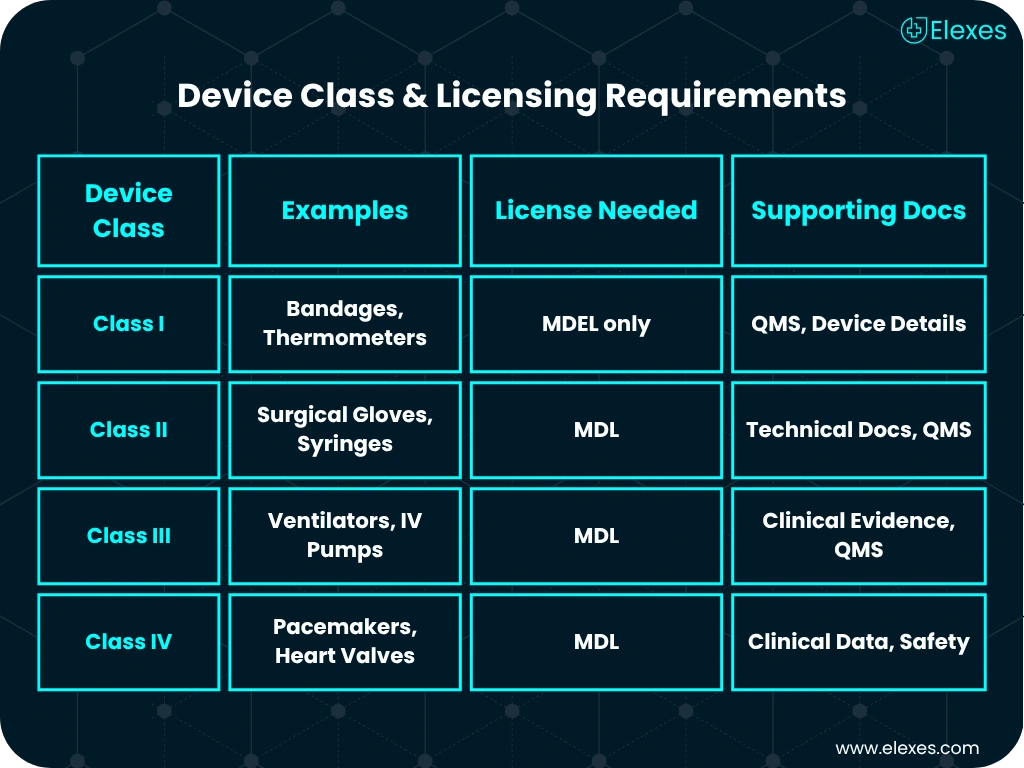
Health Canada categorizes devices into Class I to IV. Each classification brings its own level of regulatory scrutiny, documentation, and licensing steps.
If you’re importing, distributing, or manufacturing Class I devices, you’ll need an MDEL. Unlike the Medical Device License (MDL), this license focuses on company activities.
Products marketed in Canada must comply with both English and French language requirements, alongside compliance with Canadian Standards Association (CSA) and ISO standards for safety and performance.

PC: Canva
Our Health Canada consulting services include:
⦿ Classification determination and regulatory pathway strategy
⦿ Medical Device License (MDL) applications for Class II, III, IV
⦿ MDEL guidance for Class I devices, importers, and distributors
⦿ Quality Management System (QMS) aligned with MDSAP
⦿ Clinical evidence review & gap analysis (for Class III/IV)
⦿ Labeling, Instructions for Use (IFU), and UDI compliance
⦿ Preparation of Technical Files and Safety/Effectiveness Data
⦿ Communication with Health Canada on behalf of clients
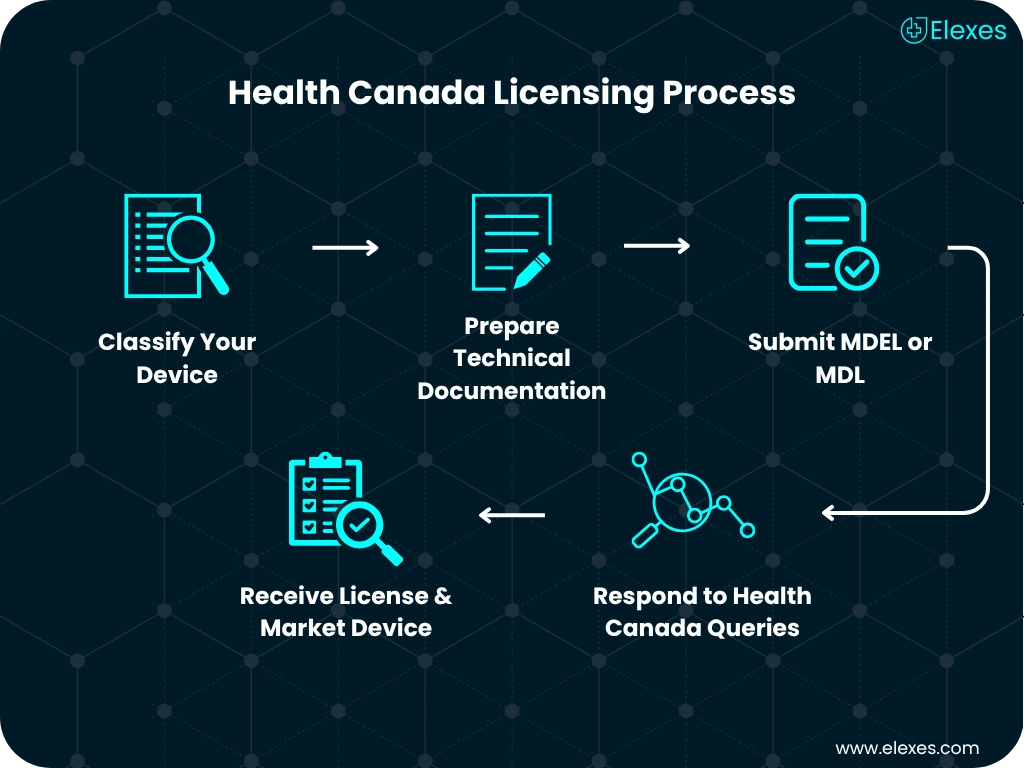
⦿ Evaluate your device’s intended use and risk profile
⦿ Determine whether MDL or MDEL is required
⦿ Map out a submission timeline that fits your launch goals
⦿ Create compliant documentation per CMDR
⦿ Submit MDL/MDEL with Health Canada
⦿ Respond to deficiency letters or additional information requests
⦿ Help with Annual License Renewal processes
⦿ PMS (Post-Market Surveillance) and Incident Reporting
⦿ Label updates or device modifications requiring new filings

PC: Canva
With 50+ clients licensed in Canada, Elexes combines regulatory experience, engineering insights, and strategic foresight to simplify your path to compliance. Our Health Canada regulatory consultants don’t just review documents; we act as your regulatory partner from planning to market launch.
Take the guesswork out of Canadian compliance. Partner with Elexes’ Health Canada consultants to get licensed faster and smarter.
Yes. Depending on the class of the device, you will need either a Medical Device License (MDL) or a Medical Device Establishment License (MDEL).
The timeline varies by class. Class II–IV MDLs may take 45–120 days, while MDELs typically process in 20 business days.
Yes, especially for Class II–IV devices. MDSAP certification is required to demonstrate QMS compliance.
Stay compliant with industry regulations and standards.
Achieve regulatory success with Elexes, all within your budget.
Experience timely results with our efficient services.
We offer 100% confidentiality understanding how critical the data is for you.
We offer different services that will help you not only keep your product well in boundaries of regulations but also speed up the entire approval process. Some of these services are -








CEO Masterlink, Arizona

CEO Novasignal, Los Angeles

President ViDava, Florida

Sr. Exe Treedental, Hong Kong

Manager Outset Medical, California

CTO Jana Care, Massachusetts

MD Blackrock Pharma, England

VP Regulatory AliveCor, California

Owner Liz Inc., Arizona

CEO Radformation, New York


























Never miss out on any important update on the regulatory & compliance industry across the globe. Subscribe to our newsletter now.
Copyright 2025, Elexes Medical Consulting Pvt. Ltd. All Rights Reserved
Working Hours : Monday to Friday 9:00 AM - 7:00 PM