
Compliance Assurance
Stay compliant with industry regulations and standards.
Cost-effective
Achieve regulatory success with Elexes, all within your budget.

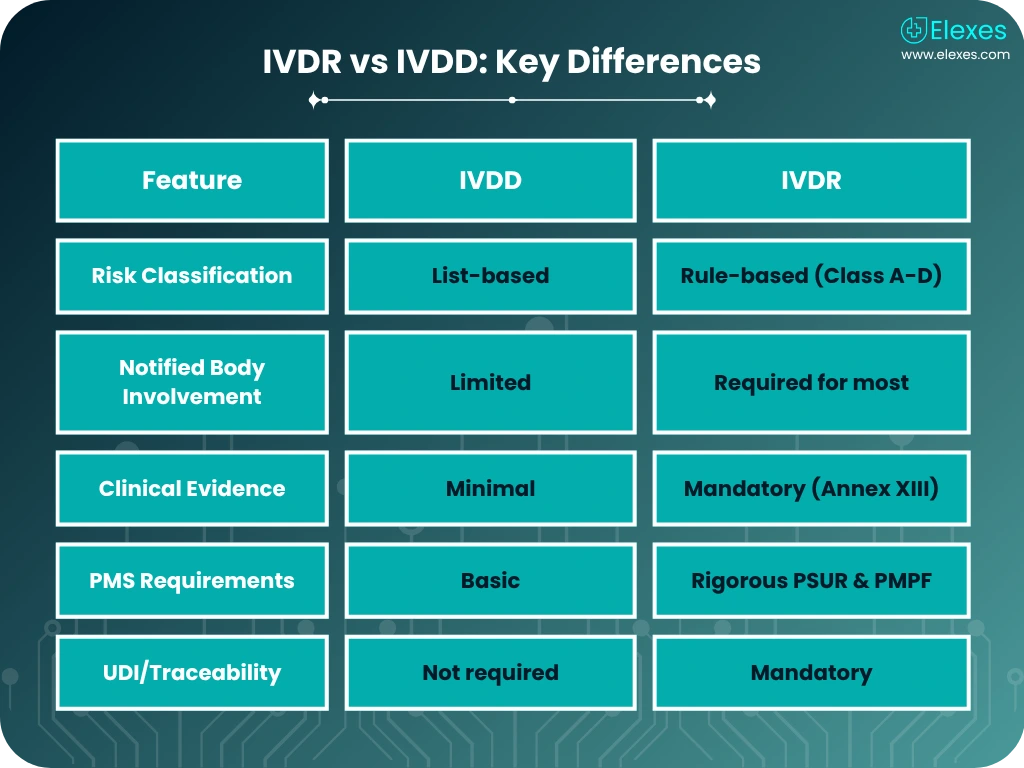
As the EU In Vitro Diagnostic Regulation (IVDR) replaces the IVDD, IVD manufacturers must navigate complex new requirements. Elexes provides tailored IVDR regulatory consulting services to ensure timely CE marking and post-market compliance under EU 2017/746.
From classification to performance evaluation and notified body liaison, our team helps you stay compliant without disrupting innovation. Up to 80% of IVDs now require Notified Body involvement under IVDR, we ensure you’re audit-ready from Day 1.
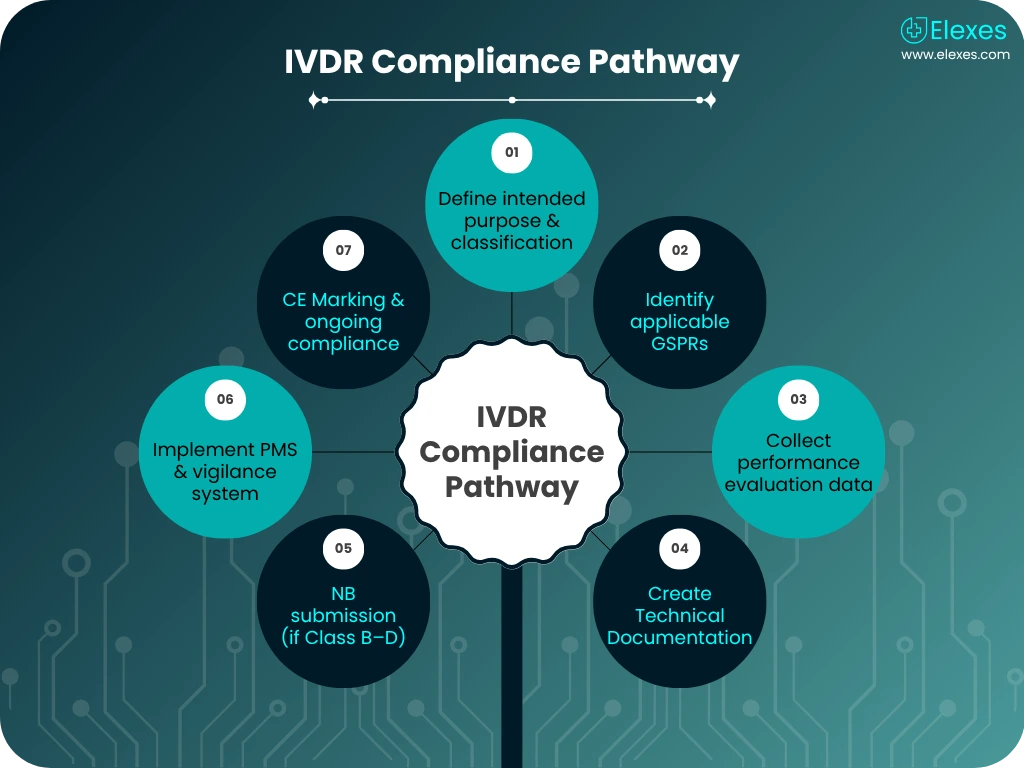
The IVDR is more rigorous than ever before, making expert support essential. Our IVDR expert services are designed to guide you from initial planning through certification and beyond.
We help you determine the correct risk class (Class A-D), ensuring compliance with Annex VIII and alignment with General Safety & Performance Requirements (Annex I).
We assist in compiling robust scientific validity, analytical, and clinical performance data in line with Annex XIII. Our support includes setting up performance study protocols and clinical evidence evaluation.
Our team prepares your complete Technical File, structured to meet IVDR Annex II/III requirements. We also support notified body submissions and responses to deficiency letters.

You get more than just documentation, we offer end-to-end strategy, execution, and continuous support.
⦿ Deep understanding of EU IVDR 2017/746
⦿ Experience with EU Notified Bodies for Class B–D IVDs
⦿ Expertise in PMS plans, PSURs, and trend reporting
⦿ Support for legacy products transitioning from IVDD
⦿ Cross-functional team of scientists, QA/RA professionals, and former auditors
⦿ Classification & conformity route selection
⦿ Intended purpose definition & clinical claim alignment
⦿ GSPR checklist completion
⦿ Clinical performance study planning
⦿ Evaluation report drafting (PER)
⦿ Literature reviews and post-market clinical follow-up
⦿ Gap analysis & remediation
⦿ Labeling & IFU review
⦿ IVDR-compliant QMS implementation and updates

Elexes goes the extra mile to support your product lifecycle, helping you prepare for audits, post-market activities, and ongoing surveillance under IVDR.
Partner with us for expert support in:
⦿ Periodic Safety Update Reports (PSUR)
⦿ Post-market performance follow-up (PMPF)
⦿ Vigilance reporting & trend analysis
Ready to achieve IVDR compliance without the guesswork? Let Elexes be your partner from strategy to certification!
The IVDR (EU 2017/746) is the new regulation for in vitro diagnostic medical devices, replacing IVDD and requiring stricter compliance.
The deadline depends on device classification and certification status. All IVDs must comply by May 2027 or earlier.
Yes, for most Class B–D devices. Class A (non-sterile) may be self-certified.
The Performance Evaluation Report (PER) includes scientific validity, analytical performance, and clinical performance data for the device.
Stay compliant with industry regulations and standards.
Achieve regulatory success with Elexes, all within your budget.
Experience timely results with our efficient services.
We offer 100% confidentiality understanding how critical the data is for you.
We offer different services that will help you not only keep your product well in boundaries of regulations but also speed up the entire approval process. Some of these services are -








CEO Masterlink, Arizona

CEO Novasignal, Los Angeles

President ViDava, Florida

Sr. Exe Treedental, Hong Kong

Manager Outset Medical, California

CTO Jana Care, Massachusetts

MD Blackrock Pharma, England

VP Regulatory AliveCor, California

Owner Liz Inc., Arizona

CEO Radformation, New York


























Never miss out on any important update on the regulatory & compliance industry across the globe. Subscribe to our newsletter now.
Copyright 2025, Elexes Medical Consulting Pvt. Ltd. All Rights Reserved
Working Hours : Monday to Friday 9:00 AM - 7:00 PM