
Compliance Assurance
Stay compliant with industry regulations and standards.
Cost-effective
Achieve regulatory success with Elexes, all within your budget.
Have a groundbreaking medical device with no clear predicate? Our De Novo consulting services are designed to help you secure market entry with confidence. The De Novo pathway is a critical FDA process for novel Class I or II devices that don’t qualify under the 510(k) route. At Elexes, we help you navigate every step from pre-submission to approval, accelerating your product’s time to market while ensuring full compliance.
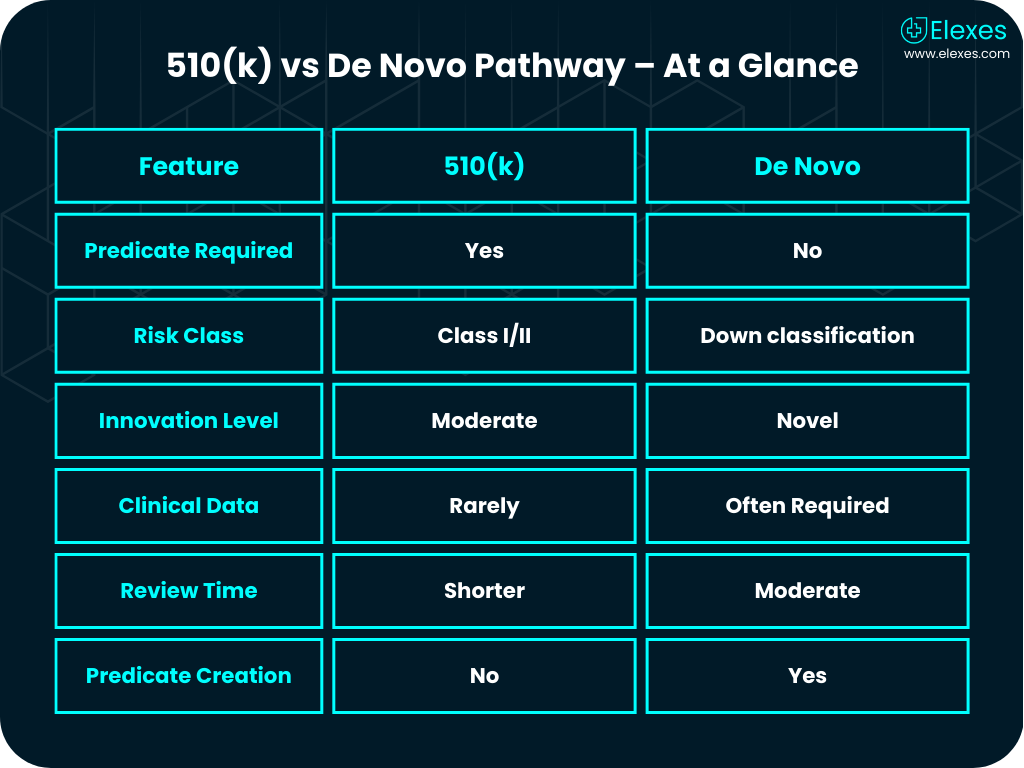
The De Novo pathway is the FDA’s solution for novel medical devices with no substantially equivalent (SE) predicate. It allows classification of low to moderate risk devices into Class I or II. This classification becomes the predicate for future 510(k) submissions.
If your 510(k) was rejected due to “Not Substantially Equivalent” or you already know there’s no existing predicate, De Novo is your best route.
It gives first movers a unique advantage. You not only get FDA approval but also set a predicate for future competitors.
Unlike 510(k), De Novo demands robust evidence including clinical and non-clinical data for risk analysis, performance, and safety.

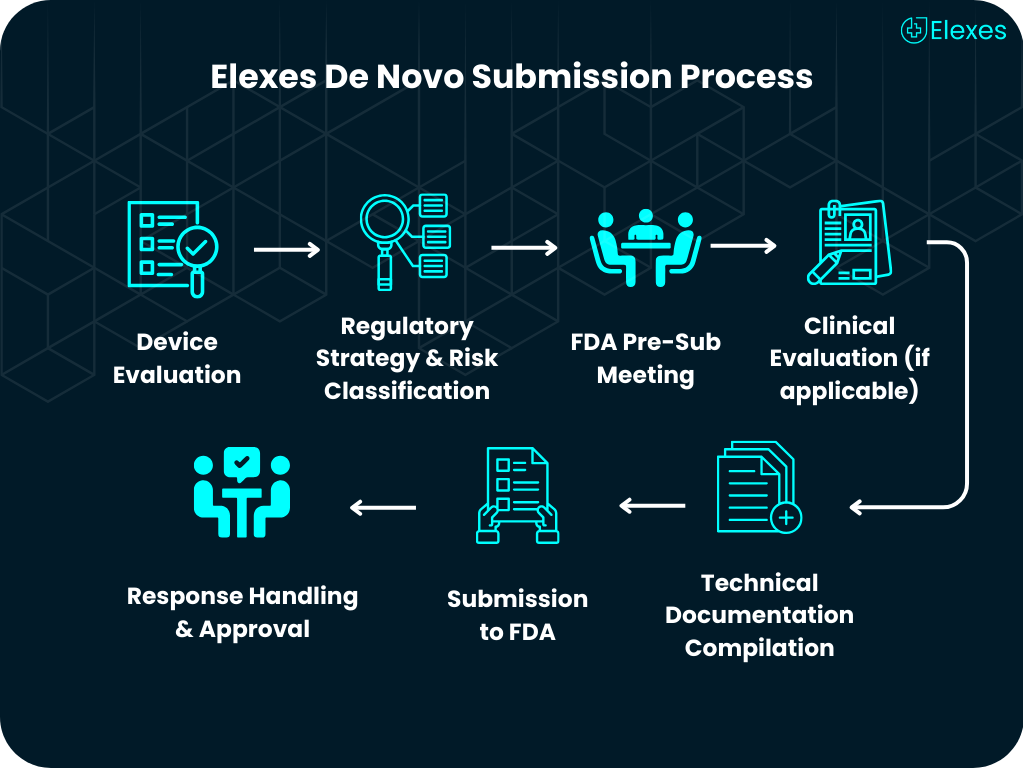
⦿ Comprehensive Device Assessment: We determine if De Novo is the best pathway for your product.
⦿ FDA Pre-Submission Support: Strategize communication with FDA to avoid future delays.
⦿ Clinical Strategy & Study Design: Plan human or animal studies aligned with FDA expectations.
⦿ Risk-Based Documentation: Help with benefit-risk analysis, special controls, and labeling.
⦿ Complete De Novo Submission Preparation: From formatting to eSTAR requirements.
⦿ Regulatory Representation: Act as your US Agent and support during FDA review.
⦿ Post-Approval Compliance: We stay with you to ensure ongoing regulatory support.
⦿ Strategy for breakthrough software, combination products, and wearables
⦿ Customized submission planning and FDA liaison services
⦿ Clinical study alignment with ISO 14155 and FDA GCP guidelines
⦿ Pre-submission package, risk management, and labeling reviews
⦿ Support with De Novo request, benefit-risk narrative, and summary reports
⦿ Device description, intended use, and indications alignment
⦿ In-house team of regulatory scientists, engineers, and clinicians
⦿ Experience with AI-based diagnostics, surgical devices, and mobile health apps
⦿ Seamless collaboration with legal, marketing, and clinical teams
Click here to download the full De Novo Submission Checklist
With Elexes, you’re not just getting regulatory support, you’re setting the benchmark for future devices. Let us help you gain approval through the De Novo classification request and position your innovation for long-term success. Click here to know what our clients have to say about us.
The De Novo pathway is a regulatory route offered by the FDA for novel medical devices without a suitable predicate. It allows classification into Class I or II.
If no substantially equivalent device exists for your innovation, and your product poses low to moderate risk, a De Novo request is the appropriate route.
On average, the FDA review process for De Novo submissions takes 150 to 180 calendar days, depending on complexity and data requirements.
Stay compliant with industry regulations and standards.
Achieve regulatory success with Elexes, all within your budget.
Experience timely results with our efficient services.
We offer 100% confidentiality understanding how critical the data is for you.








CEO Masterlink, Arizona

CEO Novasignal, Los Angeles

President ViDava, Florida

Sr. Exe Treedental, Hong Kong

Manager Outset Medical, California

CTO Jana Care, Massachusetts

MD Blackrock Pharma, England

VP Regulatory AliveCor, California

Owner Liz Inc., Arizona

CEO Radformation, New York


























Never miss out on any important update on the regulatory & compliance industry across the globe. Subscribe to our newsletter now.
Copyright 2025, Elexes Medical Consulting Pvt. Ltd. All Rights Reserved
Working Hours : Monday to Friday 9:00 AM - 7:00 PM