
Compliance Assurance
Stay compliant with industry regulations and standards.
Cost-effective
Achieve regulatory success with Elexes, all within your budget.

PC: Canva
ISO 13485 certification is the global benchmark for quality management in the medical device and IVD industry. At Elexes, we provide end-to-end support to help you achieve and maintain ISO 13485 certification with confidence. Whether you are a startup or an established manufacturer, our consultants streamline every step of your QMS implementation and certification process.
ISO 13485:2016 is not just a quality system, it's a strategic compliance asset. This standard helps demonstrate your organization’s ability to consistently meet regulatory requirements and customer expectations.
ISO 13485 is often a prerequisite for CE Marking, Health Canada licensing, and FDA QMSR alignment. It opens the door to major global markets.
A well-established QMS aligned with ISO 13485 supports efficient product design, risk management, and verification & validation processes.
This certification strengthens your organization’s ability to identify, assess, and mitigate risks, reducing recalls and increasing patient safety.

PC: Canva
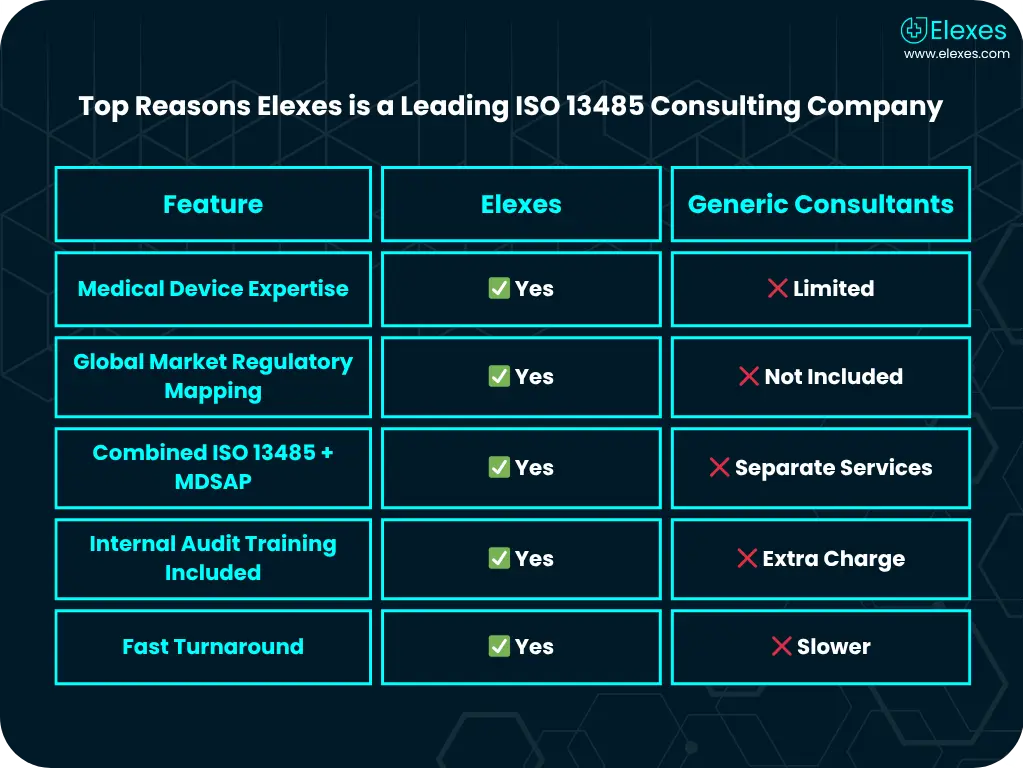
Elexes offers comprehensive ISO 13485 consulting services tailored to your product type, market goals, and current QMS maturity level.
⦿ Gap analysis and ISO 13485 readiness assessment
⦿ QMS documentation development or remediation
⦿ SOPs aligned with ISO 13485:2016 and country-specific regulations
⦿ Internal audits and management review training
⦿ Support during notified body or registrar audits
⦿ Transition assistance for ISO 13485:2003 to 2016
⦿ ISO 13485 & MDSAP harmonization support
Learn more about our MDSAP certification services
⦿ QMS from scratch
⦿ Phase-wise implementation
⦿ Document control and CAPA setup
⦿ IEC 62304 & ISO 14971 integration
⦿ Validation of software QMS components
⦿ IVDR and MDR aligned documentation
⦿ Localization of QMS for multi-country compliance
⦿ Audit preparation and representation
⦿ Supplier and subcontractor compliance
Explore our IVDR consulting and SaMD services

PC: Canva
We don’t just guide you, we partner with you. From QMS design to regulatory inspections, Elexes offers turnkey solutions to achieve ISO 13485 certification faster and smoother.
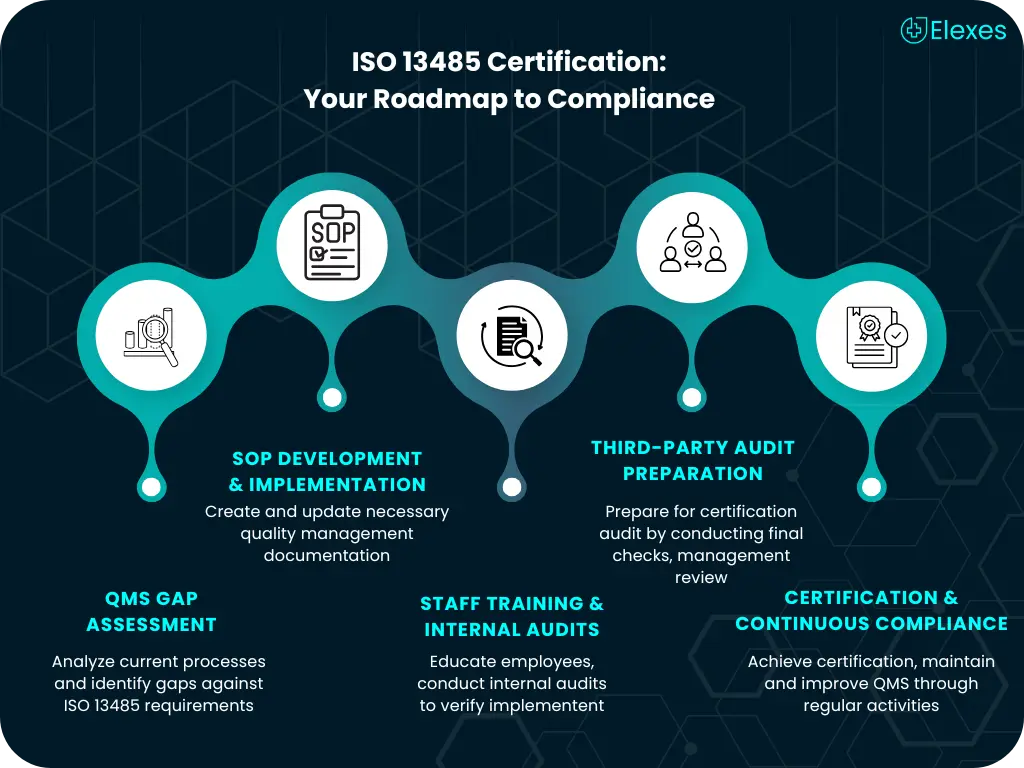
ISO 13485 certification is a globally recognized standard for QMS in the medical device industry. It ensures compliance with regulatory requirements and product consistency.
On average, ISO 13485 certification takes 4–6 months depending on your existing QMS maturity, document readiness, and team training.
Yes, ISO 13485 is typically required as part of the conformity assessment for CE marking under the EU MDR and IVDR.
Stay compliant with industry regulations and standards.
Achieve regulatory success with Elexes, all within your budget.
Experience timely results with our efficient services.
We offer 100% confidentiality understanding how critical the data is for you.
We offer different services that will help you not only keep your product well in boundaries of regulations but also speed up the entire approval process. Some of these services are -








CEO Masterlink, Arizona

CEO Novasignal, Los Angeles

President ViDava, Florida

Sr. Exe Treedental, Hong Kong

Manager Outset Medical, California

CTO Jana Care, Massachusetts

MD Blackrock Pharma, England

VP Regulatory AliveCor, California

Owner Liz Inc., Arizona

CEO Radformation, New York


























Never miss out on any important update on the regulatory & compliance industry across the globe. Subscribe to our newsletter now.
Copyright 2025, Elexes Medical Consulting Pvt. Ltd. All Rights Reserved
Working Hours : Monday to Friday 9:00 AM - 7:00 PM