Degradation Kinetics of Biomedical Polymers: Modeling Methods for Implantable Devices
The long-term performance of implantable medical devices depends strongly on how their materials behave once placed inside the human body. Among these materials, biomedical polymers play a central role in ensuring safety, stability, and clinical effectiveness. Understanding how these polymers degrade is essential because it directly affects the success of devices such as drug delivery systems, sutures, scaffolds, and stents.
A major challenge for scientists and engineers is establishing a reliable connection between in-vitro accelerated aging results and actual in-vivo degradation patterns. Even though in-vitro studies shorten testing timelines, they cannot fully mimic the intricate physiological environment inside the body. As a result, degradation kinetics observed in the laboratory often do not match long-term in vivo behavior.
To overcome this gap, researchers are increasingly using mechanistic modeling strategies combined with the Arrhenius equation to predict degradation patterns with greater accuracy.
Mechanisms of Biodegradable Polymer Degradation
Among the biodegradable polymers, poly-L-lactic acid (PLLA) and poly(ε-caprolactone) (PCL) are the most common and the primary reason for their application in implantable devices is the slow degradation of these polymers. The two mechanisms of degradation mainly involved with implantable devices are hydrolytic and oxidative degradation.
1. Hydrolytic Degradation
A hydrolytic reaction occurs when water molecules break the ester bonds within the polymer backbone. This process causes chain scission and a gradual decrease in molecular weight. Many biodegradable polymers are particularly sensitive to hydrolytic processes, which makes this mechanism one of the most widely studied.
2. Oxidative Degradation
Oxidative degradation can be viewed as a consequence of an immune response where the production of reactive oxygen species is the main energy source. Material characteristics like crystallinity, molecular weights, and polymer morphologies, in addition to environmental conditions like pH, temperature, and enzymatic activity, are the main determinants of the rates of both hydrolytic reactions and oxidative degradation.
Kinetic Modeling of Polymer Degradation Using the Arrhenius Equation
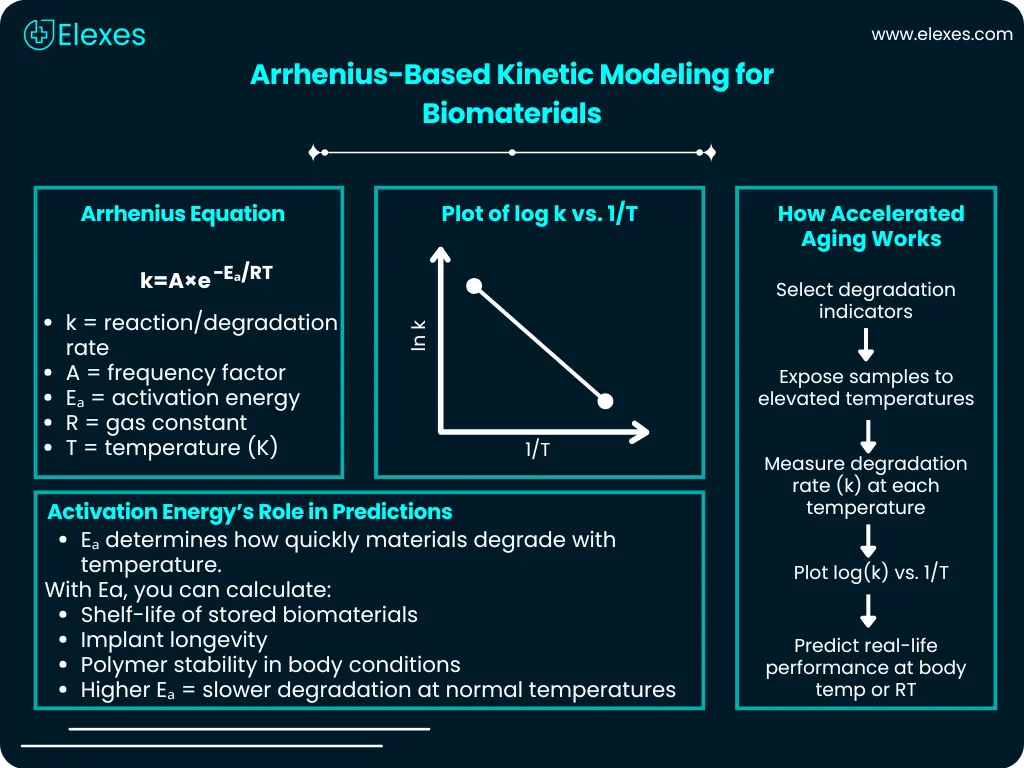
The Arrhenius equation has been an indispensable tool ever since it was introduced for predicting the degradation kinetics of biomedical and biodegradable polymers:
k=A×e−Eₐ/RT
Here, k is the reaction rate constant, A is the frequency factor, Eₐ is the activation energy, R is the ideal gas constant, and T is the absolute temperature.
By conducting accelerated aging tests under high-temperature conditions along with plotting Arrhenius curves (log k versus 1/T), one can derive activation energy and foresee long-term in-vivo degradation timeframes. Nonetheless, this method is based on the assumption that temperature only accelerates degradation and does not affect the degradation mechanism itself. This assumption may not hold true for complex polymers such as PLLA and PCL, whose degradation mechanisms can shift under physiological conditions.
The inclusion of the Arrhenius equation within mechanistic modeling improves prediction accuracy but still requires careful interpretation when applied to real-world implanted devices.
Mechanistic Models Connecting In-Vitro and In-Vivo Data
Researchers are now applying mechanistic modeling frameworks that merge different data sources. They use these models to combine in-vitro hydrolytic reactions, temperature-dependent kinetic parameters, and biological reactions, thus, being able to precisely predict polymer behavior in the body.
PLLA orthopedic screws studies showed that in-vivo degradation always takes 1.5 to 2 times longer than forecasted through Arrhenius equation calculations. Water limitation and tissue buffering were found to be the main factors that caused this difference. Similar trends have been reported for PCL scaffolds, where oxidative degradation played a more significant role than expected from controlled lab environments.
With the support of machine learning and regression-based modeling techniques, mechanistic models now provide more reliable predictions and greater alignment with regulatory expectations under FDA and ISO 10993 standards.
Future Directions in Polymer Degradation Modeling
The combination of mechanistic modeling, biological data from real-world environments, and AI-powered simulation is the primary reason for the shift in the process of implantable medical devices design and evaluation. These superior modeling techniques permit the researchers to conduct simulations of several years of in-vivo aging in just a matter of weeks, making the material selection process significantly easier and thus lessening the demand for experimental animal tests.
Predictive modeling based on hydrolytic reactions and the Arrhenius equation supports faster regulatory approvals and ensures that biomedical polymers used in next-generation devices remain safe, durable, and clinically effective over their entire intended lifespan.
As the medical device industry continues to evolve, expertise in polymer degradation modeling will remain a critical component of both scientific development and regulatory compliance.