Accelerated Aging vs Real-Time Aging for Implantable Devices: Understanding Predictive Correlation Using the Arrhenius Model
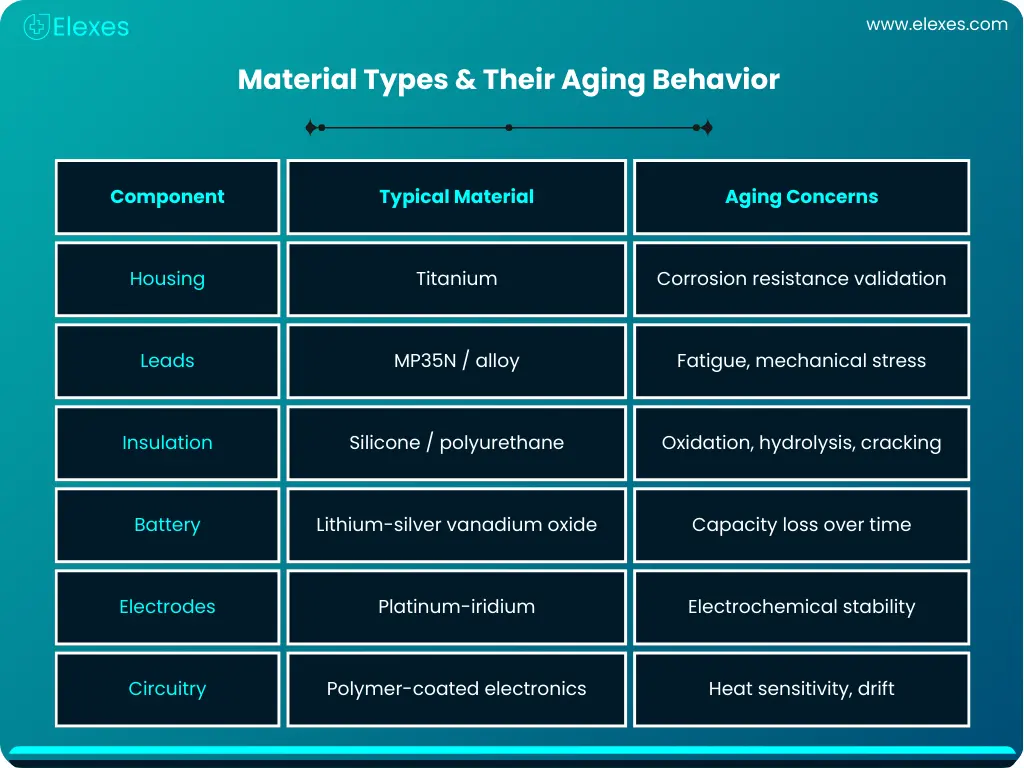
Any implanted medical device must have long-term dependability. For high-risk implants such as an implantable AICD (Automated Implantable Cardioverter Defibrillator), demonstrating predictable long-term performance is essential for regulatory approval and patient safety. Manufacturers typically rely on a combination of accelerated aging and real-time aging to estimate device durability. When paired with kinetic models such as the Arrhenius model, these approaches help predict how materials, polymers, and electronic components may behave over years of implantation.
What Is Real-Time Aging for Implantable Devices?
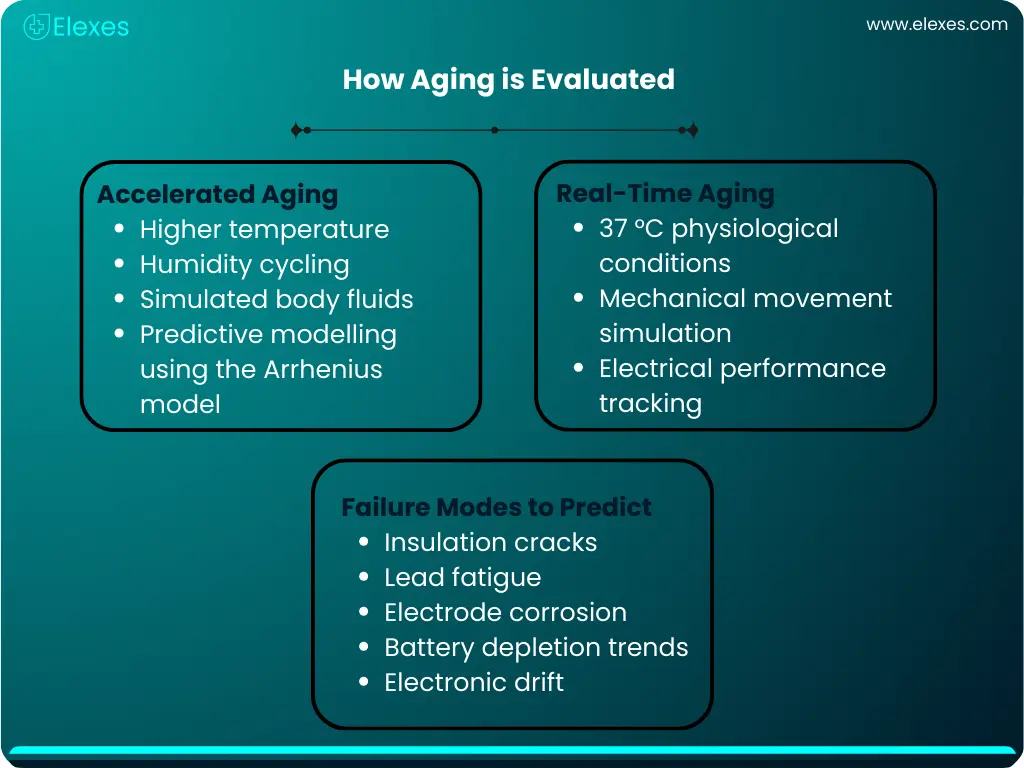
Real-time aging exposes the device to actual use-case conditions such as physiological temperature (around 37 °C), exposure to simulated body fluids, and mechanical stresses corresponding to daily patient activity.
For an implant like an implantable AICD, real-time aging provides the truest representation of long-term performance, capturing changes such as:
⦿ Polymer degradation
⦿ Battery depletion trends
⦿ Mechanical fatigue
⦿ Insulation integrity
⦿ Electrode stability
Although this method yields the most accurate results, it requires months or years to complete.
What Is Accelerated Aging and Why Is It Used?
Accelerated aging speeds up degradation by exposing the device or material to elevated temperatures, humidity, or mechanical loads. This helps predict long-term behaviour within weeks or months.
Standards like ASTM F1980 use principles of the Arrhenius model and Q10 methodology to define temperature-driven acceleration factors.
For polymeric components of implantable devices, accelerated aging helps evaluate time-dependent changes such as:
⦿ Hydrolysis
⦿ Chain scission
⦿ Oxidation
⦿ Embrittlement
⦿ Additive migration
For high-reliability products like implantable AICDs, these data points form the foundation for long-term safety claims, shelf-life determinations, and regulatory submissions.
Predictive Correlation Between Accelerated and Real-Time Aging
The key challenge in device longevity studies is correlating accelerated aging results with actual real-time behaviour. By assuming a predictable relationship between temperature and reaction rate, the Arrhenius model makes it possible to estimate long-term performance from short-term tests. Implantable systems made of multiple materials, however, may display intricate degradation processes.
Robust correlation frameworks often include:
⦿ Running parallel accelerated and real-time aging studies
⦿ Defining measurable degradation endpoints (tensile strength, electrode impedance, fatigue cycles, polymer mass loss)
⦿ Fitting accelerated data to the Arrhenius model or variable Q10 curves
⦿ Validating predictions at real-time checkpoints
⦿ Using these validated curves to forecast 5–10 year implant performance
For devices such as implantable AICDs, this process supports critical decisions such as lead redesign, insulation selection, and battery chemistry optimization.
Simulation-Enhanced Studies for Implantable Devices
Simulation models and accelerated aging data are combined in contemporary validation techniques. Some examples of predictive frameworks are:
⦿ Finite-element analysis of stress and strain
⦿ Kinetic modelling based on the Arrhenius model
⦿ Moisture diffusion and hydrolysis modelling
⦿ Fatigue life estimation under physiological load
⦿ Electrical performance drift modelling for implantable AICDs
This hybrid approach helps predict device performance more accurately over long implant durations and strengthens regulatory justification for lifetime claims.
Why Accelerated Aging Matters for Implantable AICDs
For an implantable AICD, long-term reliability is directly tied to patient survival. Predicting failure modes such as insulation cracking, electrode corrosion, or connector fatigue is essential. Using accelerated aging in combination with real-time aging and the Arrhenius model allows manufacturers to:
⦿ Reduce time to market
⦿ Generate evidence for regulatory submissions
⦿ Support shelf-life and service-life claims
⦿ Optimize materials and design choices
⦿ Build strong scientific justification for longevity claims
Conclusion
Both accelerated aging and real-time aging are indispensable tools in validating long-term performance of implantable medical devices, including implantable AICDs. When supported by predictive models such as the Arrhenius model, these strategies help manufacturers build reliable lifetime predictions while meeting global regulatory expectations.
At Elexes, we support manufacturers with end-to-end validation strategy, documentation, and regulatory submissions for implantable medical devices. Contact us if you need expert guidance on aging studies, lifetime modelling, or regulatory pathways.





















