Medical Device Trade: FDA Import-Export Policies for the US
Globalization has transformed trade and communication between developed and developing nations, leading to a surge in the movement of goods—including medical devices—across borders. In the healthcare sector, FDA Import-Export of medical devices has opened new opportunities for faster access to innovative technologies worldwide. While this global reach benefits manufacturers, it also comes with the responsibility to comply with the import and export regulations of each destination country.
Here are some quick tips for US import and export:
Medical Device Importing into the U.S.
All foreign firms importing medical devices (including radiation emitting devices) into the US market must fill forms and comply with the applicable U.S regulations before, during and after the import.
Every manufacturer importing into the U.S. :
- Establishment and Registration
- Device Listing
- Quality System Regulations (QSR)
- Medical Device Reporting of Adverse Events
- Medical Device Tracking Provisions (UDI, lot number, model number)
- Labelling Requirements
- Regulatory approval (510k), PMA or Premarket Notification)
- Appointed US Agent (applicable to foreign based companies)
- An Initial Importer (applicable to foreign based companies)
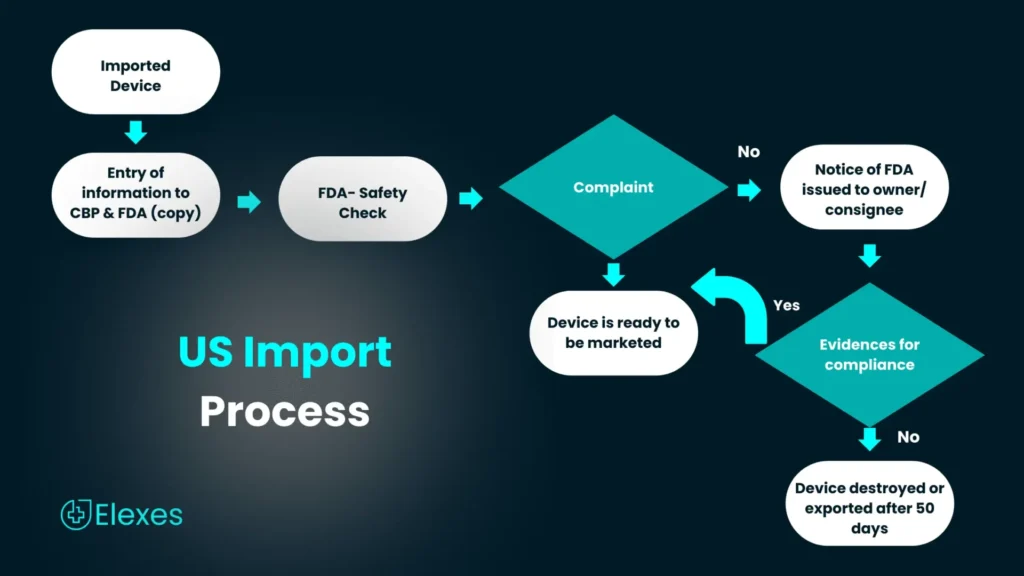
Medical devices imported into the U.S. FDA approved and must meet the requirements of CBP. Manufacturers must include appropriate information indicating compliance the FDA regulations. Products that are not compliant and which do not meet these requirements will be detained upon entry.
Establishments involved in the production and distribution of the medical devices in the U.S are required to pay the annual registration fee.
The same rules don’t apply to all. Who Must Register and List?
All the establishments can be registered using the FDA Unified Registration and Listing System (FURLS). Establishments must visit the Device Facility User Fee (DFUF) website for the payment of the user fee in order to proceed with the registration.
The annual fees for this fiscal year (2018) is $4,624. This annual registration is mandatory for facilities that need to register.

Exporting Medical Devices from the U.S. Market
Legally marketed devices in the U.S that follow export provisions as per FD&C Act can be exported to any part of the world without any prior FDA notification or approval.
Depending on the country of export, an export certificate or letter that contains proof of the regulatory and marketing status of the products in the U.S market may be required. Manufacturers exporting devices will be requested for one of the following:
All the above mentioned certificates/permit letters can be accessed through the CDRH Export Certification and Tracking System (CECATS) electronic systems in the FDA Unified Registration and Listing Systems (FDA, FURLS).
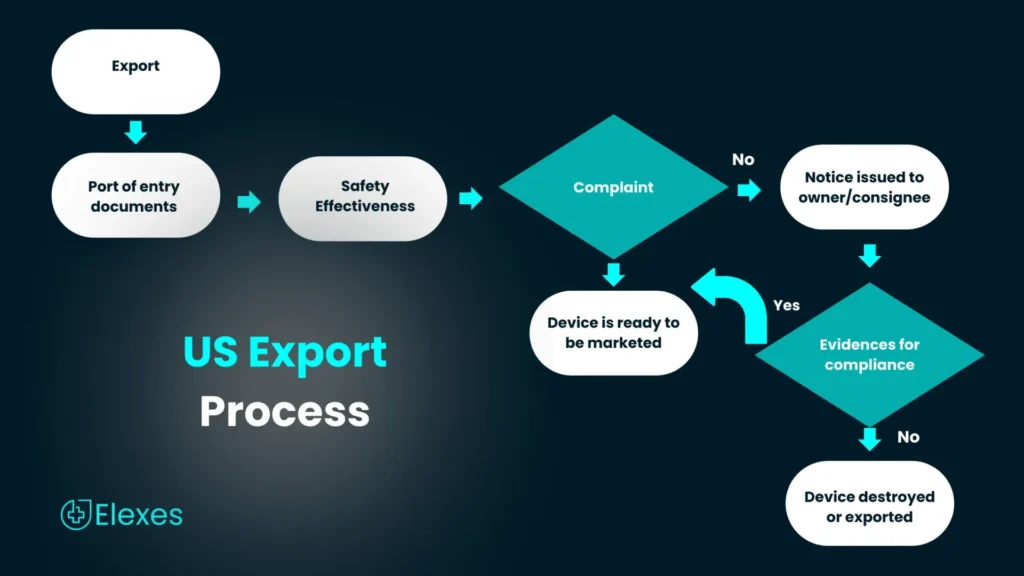
Devices exported from the U.S, should produce a CFG or other regulatory documents depending on the destination country requirements. Failure to do so could result in delayed shipment and compliance issues.
Port of Entry Requirements for obtaining a CFG
Import/Export from a regulatory standpoint is a straightforward and a well established process. However, if the right steps are not followed this process can be perpetual resulting in delayed shipment, product and revenue losses, or denial of export privileges.
Feel free to contact us at Elexes for further questions.





















