Expertise
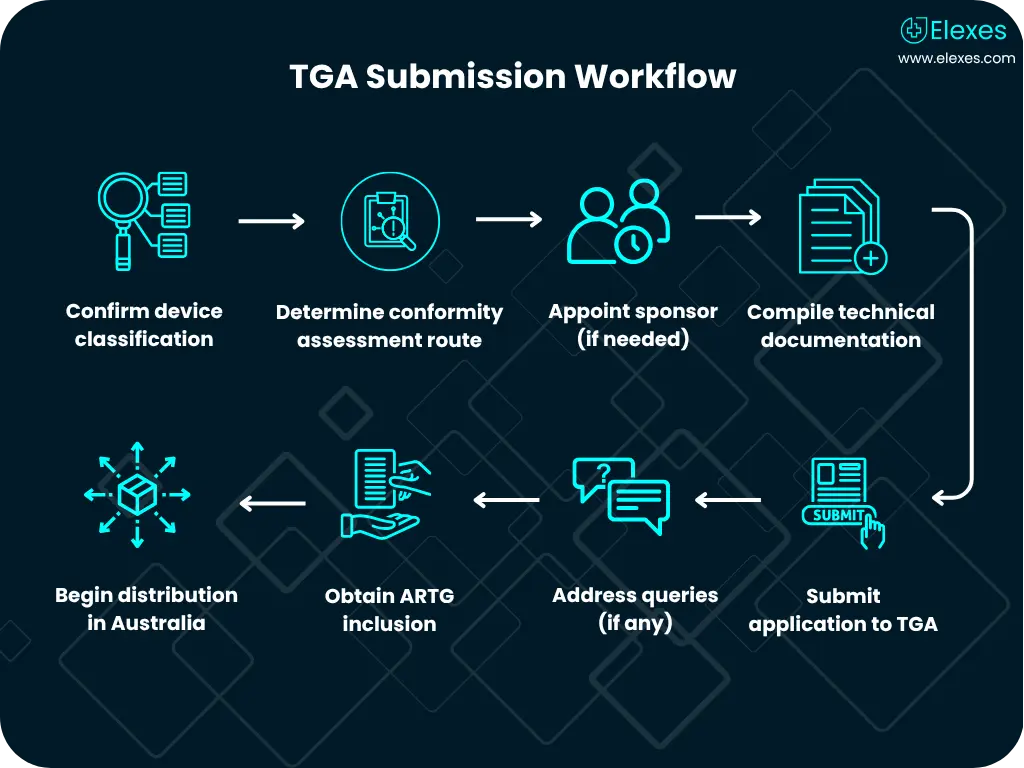
Our experts provide guidance and consultants to help you adhere to necessary regulations, ensuring your products meet all legal requirements and is compliant to all necessary standards.
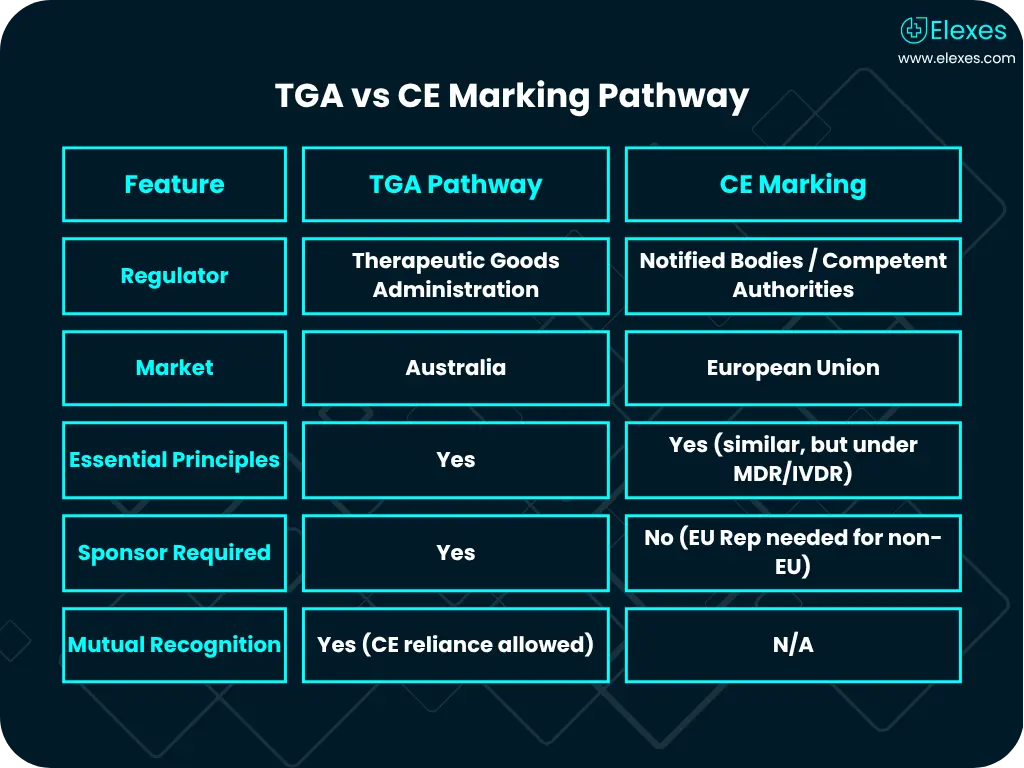
Local Regulatory Certification
At Elexes, we offer to assist in securing additional local certifications, enabling you to manufacture and sell medical devices in various markets across the globe.
Confidentiality
We offer complete confidentiality to all our clients which makes us one of the most trusted regulatory and quality compliance consulting.