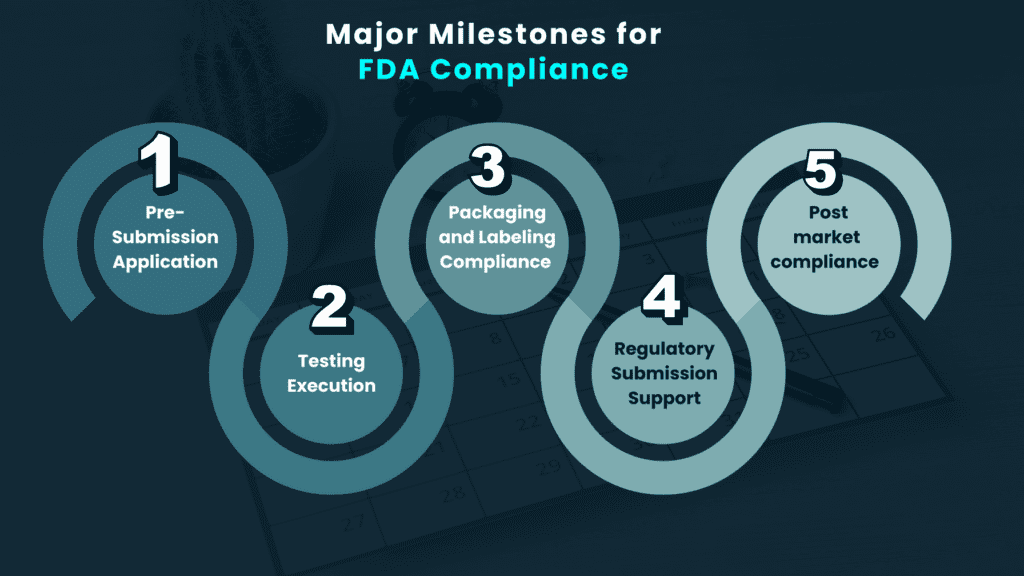
About Client
A medical device company pioneering the development of a point-of-care blood glucose monitoring device came to Elexes for expert guidance in securing a FDA 510(k) clearance for their device.
Device Overview
The client’s innovative device featured a compact design, allowing users to monitor blood glucose levels conveniently. It included essential accessories like lancets, test strips, and a user-friendly interface for seamless operation. The device came in two configurations, catering to different user preferences and needs.