Are you a Medical Device Manufacturer? an employee of a medical device company? Or perhaps someone with an interest in Medical Devices. If yes, then postmarket surveillance is something critical for you to know.
It is a well-known fact that Medical Device manufacturing is a highly cumbersome process; all your efforts pay back when your device overcomes the regulatory hurdles and you begin selling in the market. But, if you are thinking you are done with all the regulatory requirements and can sit back and relax, then you are at risk. In most cases, issues arise once the users start using the devices.
Postmarketing surveillance (PMS) is a collection of activities and processes used to monitor the performance of a Medical Device which is sold in the market. The purpose of PMS is to gather real-time data on the device to expediently identify device design or usage problems and accurately characterize the real-world device behaviour and clinical outcomes. The need for PMS arises immediately upon the commercialization of a device. PMS helps you ensure that after your product is on the market, it remains safe and effective throughout its lifecycle.
Classification of Post-marketing surveillance

Whether it be a reactive or a proactive PMS, both must feed into your quality systems, so that you can reach a value-added output after the entire PMS process. Below is a high-level representation of how PMS data can feed into a quality system.
One most common type of PMS that we regularly deal with is a complaint. Many might think that complaints are bad, but let me tell you, Complaints are not always bad! Yeah, that’s true! Complaints can be perceived as an opportunity to improve. Perfection, as we know, is an idealistic concept! Device failures are bound to occur. Hence, when complaints are received, manufacturers can understand what went wrong and where, and implement corrective actions.
All undesirable events associated with a device may not be necessarily categorized as complaints. Let’s take a look.
Consider a company X, a Medical Device Manufacturer. It is analyzing the feedback on a product. How to decide whether the feedback is a complaint or not?

For knowing when to file a complaint, when to report to the regulatory body, expert regulatory partners can be engaged. When it comes to post-marketing surveillance, regulatory bodies, like FDA and European Commission (EC) have laid out strict timelines for reporting issues related to medical devices. Hence, having dedicated personnel to stay on top of these requirements is instrumental.
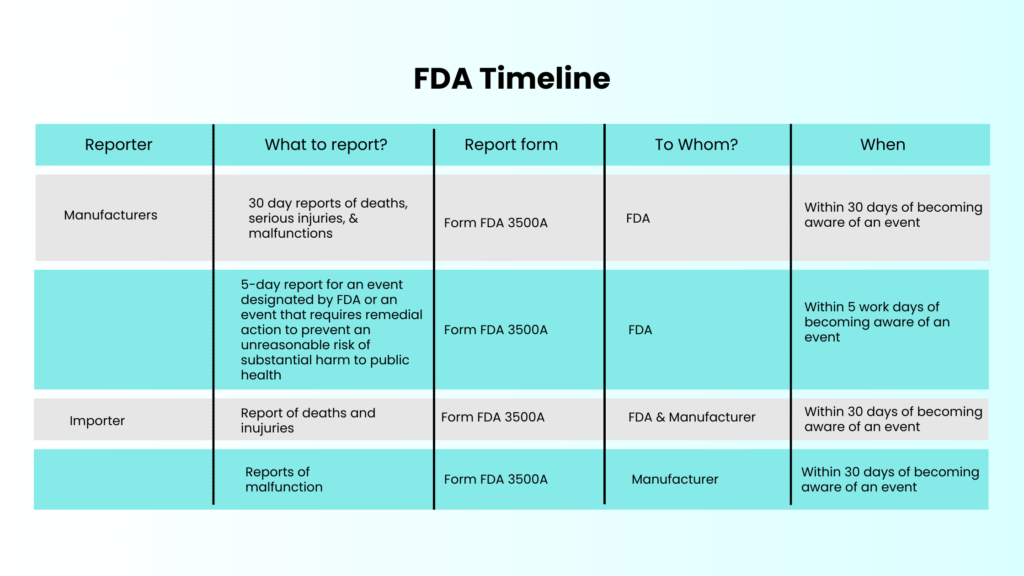
The table below presents the timeline given by the FDA and EC.

Bottom Line
In a nutshell, postmarket surveillance is an important part of a medical device lifecycle, and if handled correctly, it can help you improve your product and create devices that consistently exceed user expectations.
However, if handled incorrectly, it can have serious repercussions which can result in recalls, market withdrawals, customer dissatisfaction. Complaints and device failures insinuate that the company is not applying the proper means to manufacture the device.
This spoils the brand name and breaks the consumer’s trust and could lead to further monetary losses. ELEXES can help you in making sure that your company is not only able to efficaciously handle PMS data to derive improvements but can also pre-emptively avoid complaints or device issues by meeting all applicable regulatory and statutory requirements.






















