In the domain of Good Manufacturing Practices (GMP), a blend of crucial principles and processes (based on standards and requirements) work together to ensure the safety, quality, and consistency of products.
Compliance with GMP is not only an ethical imperative, but a legal requirement in many jurisdictions, enforced through regulatory inspections and audits to ensure strict adherence to certain critical standards.
What is GMP?
We are often asked what does GMP mean?
Good Manufacturing Practices, commonly known as GMP, is a set of guidelines and principles established to ensure the consistent quality and safety of pharmaceuticals, food products, medical devices, cosmetics, and other consumer goods.
These standards are critical to various industries, as they not only protect the health and well-being of consumers but also safeguard the reputation and success of manufacturers.
Origin of GMP
The concept of Good Manufacturing Practices can be traced back to the early 20th century when the industrialization of medical devices, food, and pharmaceutical production led to concerns for product safety and quality.
At that time, there were no standardized regulations governing the manufacturing processes and quality control measures. The absence of proper supervision led to an increase in subpar and hazardous goods within the market.
The need for more comprehensive and specific guidelines became increasingly evident, especially with the growth of several industries.
In the mid-20th century, various countries and international organizations began to develop their own GMP standards, leading to a global effort to harmonize these practices.
Today, GMP is enforced by regulatory authorities worldwide, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), with one goal – “ensuring the quality and safety of products across borders”.
The Essence of GMP
GMP is essentially a set of quality assurance principles and guidelines that outline the methods, equipment, facilities, and controls required for the production of safe and high-quality products.
These guidelines cover every facet of manufacturing, spanning from the choice of raw materials to the delivery of final products.
The key objectives of GMP can be summarized as follows:

GMP ensures that products consistently meet quality standards by implementing robust quality control and quality assurance systems.
It helps in recognizing and addressing potential risks linked to the manufacturing process, materials, and products.
Protecting the health and safety of consumers by preventing contamination, adulteration, and errors in product manufacturing is another role that GMP plays.
GMP also helps in maintaining uniformity in product quality, specifications, and performance over time.
Thoroughly documenting all aspects of manufacturing to provide a transparent record of production processes is a part of GMP.
GMP motivates manufacturers to consistently evaluate and improve their processes to attain elevated standards of quality.
Key Principles of GMP

Properly designed and maintained facilities and equipment are fundamental to GMP. Manufacturers are required to establish a regulated and hygienic environment to avert contamination and uphold product integrity.
This includes:
- Regular maintenance
- Consistent cleaning
- Validation of equipment and facilities
For example: In pharmaceutical manufacturing, a GMP-compliant facility would include separate areas for handling different drug components to prevent cross-contamination. Production equipment receives regular maintenance and calibration to guarantee precision and dependability.
A skilled and trained workforce is crucial in implementing GMP effectively. Personnel should be educated on the principles of GMP, hygiene, and safety procedures. Moreover, roles and responsibilities should be well-defined, and personnel should be aware of the importance of their contributions to product quality.
For Example: In the production of magnetic resonance imaging (MRI) machines, a skilled and well-trained workforce is paramount for the effective implementation of GMP.
These complex medical devices require precision engineering and stringent quality control to ensure patient safety and diagnostic accuracy. Technicians and engineers are extensively educated on GMP principles specific to medical device manufacturing, including the importance of maintaining a sterile environment within the MRI machine’s sensitive components.
They follow strict hygiene protocols, such as wearing sterile gowns and gloves, and practice meticulous hand hygiene to prevent contamination.
Safety protocols are diligently upheld to protect personnel and maintain the equipment’s reliability.
Personnel are acutely aware that deviations from GMP standards can compromise the quality of MRI scans and patient diagnoses, underscoring the vital role they play in delivering high-quality medical devices essential for healthcare professionals and patients.
Thorough documentation plays a fundamental role in the foundation of GMP. Manufacturers must maintain records of every aspect of production including:
- Batch records
- Equipment maintenance logs
- Quality control data
This documentation provides a clear trail of actions taken during manufacturing and allows for traceability and accountability.
For example: A pharmaceutical company producing a new medication would maintain detailed records of the ingredients used, the manufacturing process, and quality control tests conducted at various stages. This ensures that any issues can be traced back to their source for investigation and resolution.
Quality control is central to GMP and involves rigorous testing and inspection of raw materials, intermediate products, and finished goods. These tests ensure that products meet established quality specifications and are safe for consumption or use.
For example: In the production of insulin infusion pumps, adherence to GMP relies significantly on the critical role of quality control.
Throughout the production process, from the procurement of components to the assembly of the final device, a battery of tests and inspections are conducted. Raw materials, such as medical-grade plastics and electronic components, undergo thorough quality checks to verify their conformity to established specifications.
During assembly, each pump is subjected to stringent inspections to guarantee precise dosage delivery and safety features like alarm systems. Prior to distribution, a final quality assessment ensures that every insulin infusion pump meets the GMP-mandated standards, assuring healthcare providers and diabetic patients of a reliable and safe medical device for managing insulin therapy.
Assessing and validating the integrity of raw materials and components is an essential component of GMP. Manufacturers must establish specifications for these materials, conduct supplier audits, and implement receiving inspection processes to ensure only acceptable materials are used in production.
For example: In the production of cardiac stents, adhering to GMP involves rigorous control over raw materials and components. The stainless steel or nitinol alloys used to craft stents must meet exact specifications to ensure durability and biocompatibility within the human body.
Manufacturers establish precise standards for these materials, conducting supplier audits to verify their compliance with GMP requirements. Upon receipt at the production facility, every batch of raw materials undergoes a comprehensive receiving inspection.
This inspection encompasses dimensional checks, material composition analysis, and surface finish assessments to confirm that only materials meeting the stringent GMP criteria are integrated into the stent manufacturing process.
Process validation involves a confirmation that a manufacturing process consistently yields products in accordance with predefined quality criteria. This requires a scientific approach, with data-driven assessments to ensure the process is robust and capable of delivering reliable results. Also, validation before the use of any equipment, software, or computer systems that are involved during any QMS activities is vital.
For example: In the manufacturing of blood glucose monitoring systems, process validation is a critical component of GMP. Before mass production begins, the manufacturing process for these devices undergoes extensive validation to ensure consistent and accurate results for patients with diabetes.
This validation includes testing the precision and reliability of the glucose measurement process, assessing the functionality of the device’s software and data management systems, and confirming the compatibility of test strips and lancets.
A robust scientific approach is employed, with meticulous data collection and analysis to verify that the manufacturing process consistently meets the predefined quality standards. Prior to production, validation of equipment, software, and computer systems used in quality management activities is also conducted to ensure the reliability and integrity of the entire quality control process.
Self-inspections are a proactive measure within GMP that allows manufacturers to assess their own compliance with GMP standards. These audits help identify areas of improvement, correct deficiencies, and maintain a state of continuous readiness for external regulatory inspections.
For example: A pharmaceutical company conducts a self-inspection of its manufacturing facility. During the audit, they discovered that some production personnel were not consistently following gowning procedures. This finding was documented, and immediate retraining was conducted to correct the issue and prevent future occurrences.

Fundamental GMP principles encompass production and process controls, serving as the bedrock for maintaining product consistency and quality. These controls encompass various elements, including the design and maintenance of equipment, monitoring of critical process parameters, and the use of validated processes and procedures.
For example: In the production of hearing aids, adherence to GMP is essential, and production and process controls are central to ensuring the quality and reliability of these medical devices. Hearing aids are intricate devices that demand meticulous engineering and stringent quality assurance processes.
The production process involves the design and maintenance of specialized equipment, such as microelectronics assembly machines and precision molding tools, to ensure the precise assembly of components.
Critical process parameters, including sound quality, amplification levels, and battery life, are continually monitored during production to guarantee that each hearing aid meets specified standards. Additionally, the use of validated processes and procedures, from the selection of materials to the final product assembly, is crucial in delivering hearing aids that consistently perform at the highest level, providing individuals with hearing impairments a better quality of life.
Are you a pharmaceutical manufacturer in search of a comprehensive manufacturing checklist? Explore this guide for a pharmaceutical manufacturing compliance checklist tailored to U.S. companies.
Handling complaints and recalls is an integral component of GMP, as it directly impacts consumer safety and product quality. When consumers report adverse reactions or issues with a product, it becomes essential for manufacturers to investigate, take corrective actions, and, in severe cases, initiate product recalls. This proactive approach helps prevent harm to consumers and protects a company’s reputation.
For example: Imagine a pharmaceutical company receiving complaints from patients about an unusual odor in a particular batch of medication. Upon investigation, it was discovered that a specific raw material supplier delivered contaminated ingredients. The company recalls all affected products, identifies an alternative supplier, and implements stricter quality control measures for incoming raw materials.
Are you struggling to handle all of the complaints you are receiving, please do not hesitate to get in touch with us. We stand prepared and eager to offer you our unwavering support and aid. You can reach out to us to be your complaint investigator.
The Importance of GMP
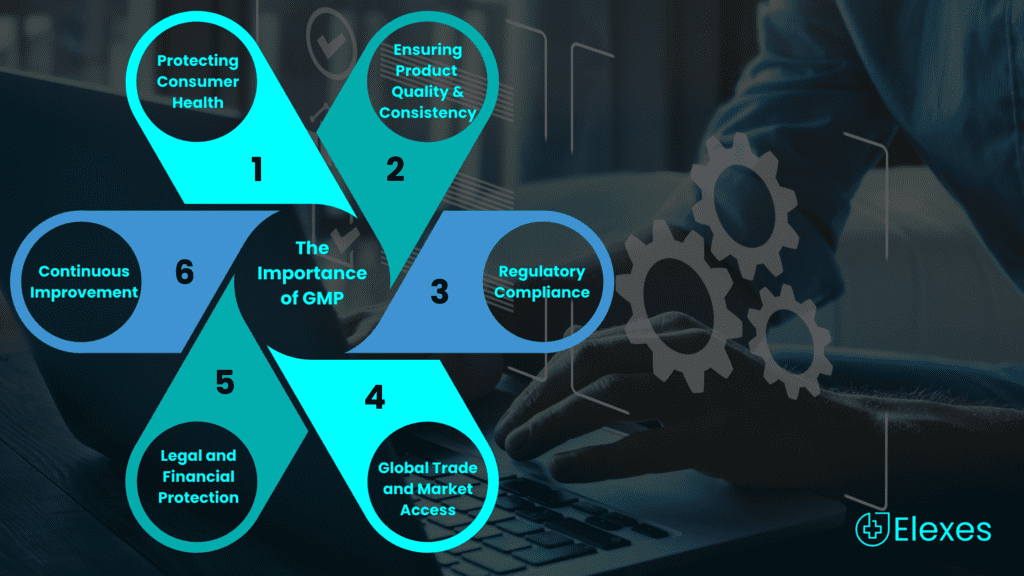
Many manufacturers often wonder why GMP is important. Let’s break it down to better understand this.
Perhaps the most significant reason for the existence of GMP is to protect the health and safety of consumers. By adhering to GMP standards, manufacturers reduce the risk of producing contaminated, substandard, or unsafe products. This holds particular significance in sectors such as pharmaceuticals and food, given the potentially severe repercussions of product defects.
In the pharmaceutical industry, GMP compliance ensures that medications are manufactured under stringent quality controls, minimizing the risk of harmful side effects or ineffective treatments. This protects patient's well-being.
GMP is instrumental in maintaining product quality and consistency. When manufacturers follow GMP guidelines, they can produce products that consistently meet specified standards. This consistency builds trust among consumers, leading to brand loyalty and enhanced market competitiveness.
Compliance with GMP is often a legal requirement in many industries, enforced by regulatory authorities. Neglecting to abide by these regulations can result in significant repercussions, encompassing product recalls and potential legal ramifications, along with harm to a company’s standing and integrity.
The FDA in the US regularly inspects pharmaceutical manufacturing facilities to ensure GMP compliance. Failure to adhere to regulations may lead to the revocation of product approvals and significant financial penalties.
In today’s interconnected global economy, adherence to GMP standards is essential for international trade. Many countries have adopted GMP guidelines as the basis for assessing the quality and safety of imported products. Having GMP certification can facilitate market access and ease the process of exporting goods.
Adhering to GMP standards reduces the risk of legal liabilities resulting from product defects, recalls, or harm to consumers. It additionally safeguards against monetary setbacks linked to penalties imposed by regulatory authorities and the expenditures incurred in addressing and rectifying compliance issues.
To foster a culture of continuous improvement, GMP encourages manufacturers to prioritize ongoing enhancements. By regularly reviewing and enhancing their processes, companies can identify opportunities for efficiency gains, cost reduction, and quality improvement.
Challenges in Implementing GMP
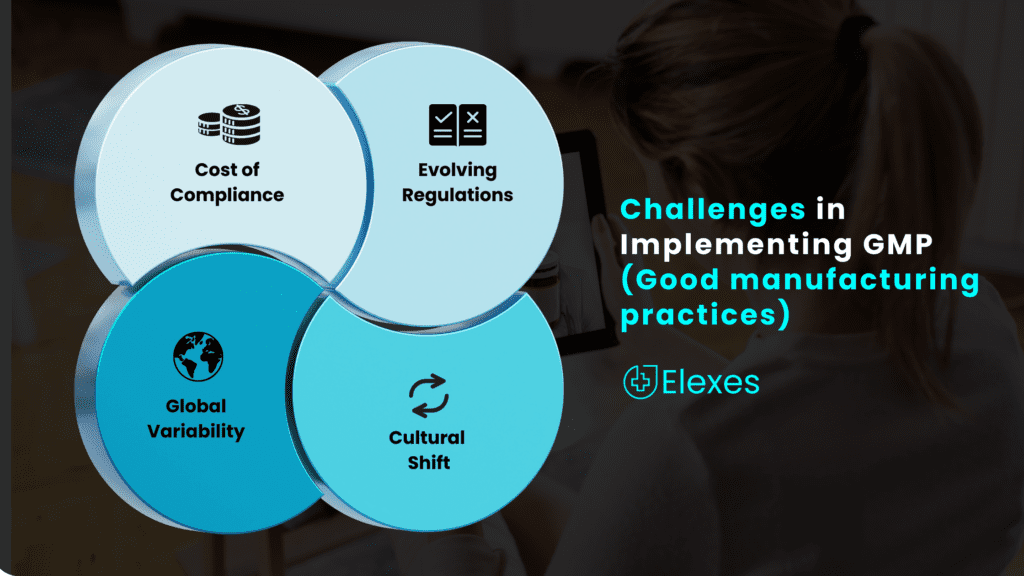
While GMP offers numerous benefits, its implementation comes with challenges that manufacturers must address:
Adhering to GMP standards often requires significant investments in facilities, equipment, personnel training, and quality control processes. Small businesses, in particular, may encounter difficulties when allocating resources to achieve complete compliance.
A small-scale cosmetics manufacturer may struggle to afford the infrastructure upgrades and specialized testing equipment needed to meet GMP requirements.
GMP regulations are not static; they evolve to keep pace with advances in technology and changes in industry practices. Manufacturers need to remain updated regarding these modifications and adjust their procedures accordingly.
Pharmaceutical companies must continually update their manufacturing processes and documentation to meet changing regulatory requirements and ensure GMP compliance.
Introducing GMP often necessitates a change in the organizational culture. Employees must fully embrace the principles of quality control and take responsibility for their roles in maintaining compliance.
In a traditional manufacturing environment where quality control was lax, transitioning to a GMP-compliant culture may require extensive training and a change in mindset among workers.
GMP standards can vary from one country or region to another, making it challenging for multinational companies to ensure consistent compliance across their operations.
A multinational food conglomerate must navigate different GMP requirements in each country it operates, necessitating a complex system for compliance management.
Want to learn more about GMP? Check our blog on GMP audit checklist | A Professional Take on it! GMP audit checklist | A Professional Take on it! to further understand GMP.
Conclusion
In conclusion, Good Manufacturing Practices (GMP) is a set of principles and guidelines that play a pivotal role in ensuring the quality, safety, and consistency of products in various industries, including pharmaceuticals, food, medical devices, and more.
GMP principles cover a wide range of aspects, from facilities and personnel to documentation and quality control, with the ultimate goal of protecting consumer health and maintaining product quality.
The importance of GMP cannot be overstated. It safeguards consumers from potentially harmful products, ensures product quality and consistency, and facilitates compliance with regulatory requirements.
GMP is also crucial for global trade and market access, enabling businesses to compete on a global scale. Furthermore, it nurtures an environment of ongoing enhancement, resulting in increased efficiency and improved manufacturing procedures.
While there are challenges in implementing GMP, including the cost of compliance and the need to adapt to evolving regulations, the benefits far outweigh the challenges.
As industries continue to evolve, GMP will remain a cornerstone of quality assurance and consumer protection, contributing to safer and higher-quality products for people around the world.
Are you considering the establishment of GMP for your company? Do not hesitate to drop us an email. We can offer guidance on the optimal path to GMP compliance.