Embarking on Good Manufacturing Practice (GMP) audits is crucial for carefully exploring your organization’s dedication to quality, safety, and meeting regulatory standards.
These audits, conducted in various industries including pharmaceuticals, medical devices, and food, serve as both a magnifying glass for regulatory procedures and a roadmap to complete compliance.
They provide a comprehensive overview of your company’s adherence to GMP regulations, revealing not only the big picture, but also the finer nuances that demand attention and improvement.
As we delve into the world of GMP audits, it’s natural to feel a sense of apprehension, but fear not – armed with the right knowledge and preparation, you can transform this process from intimidating to empowering.
In this guide, we will explore:
Let’s dive into the world of audit know-how and preparedness. Success isn’t just a goal; it’s a constant dedication to excellence.
What are GMP Audits, and Why Do They Matter?
In the realm of regulated industries, Good Manufacturing Practice (GMP) audits are very important to the authorities that ensure product quality, safety, and consistency.
But what exactly are these audits, and why are they indispensable to businesses?
What are GMP Audits?
A GMP audit is a meticulous process involving either internal or external individuals or teams who scrutinize a manufacturer’s adherence to their documented Good Manufacturing Practices.
Think of it as an integral part to a financial audit but with a twist—instead of delving into financial records, auditors delve into production processes to ensure they align with stringent quality control guidelines set by the regulatory authorities.
The Ultimate Goal
At its core, the ultimate goal of a GMP audit is crystal clear: assure that products are not only safe for use but also consistently meet the expectations of discerning customers.
In simpler terms, when you purchase a product, be it a pharmaceutical, medical device, or packaged food item, you expect it to be the same high-quality item each time you get it. GMP audits play a pivotal role in making that expectation a reality.
The Five Pillars of GMP Audits
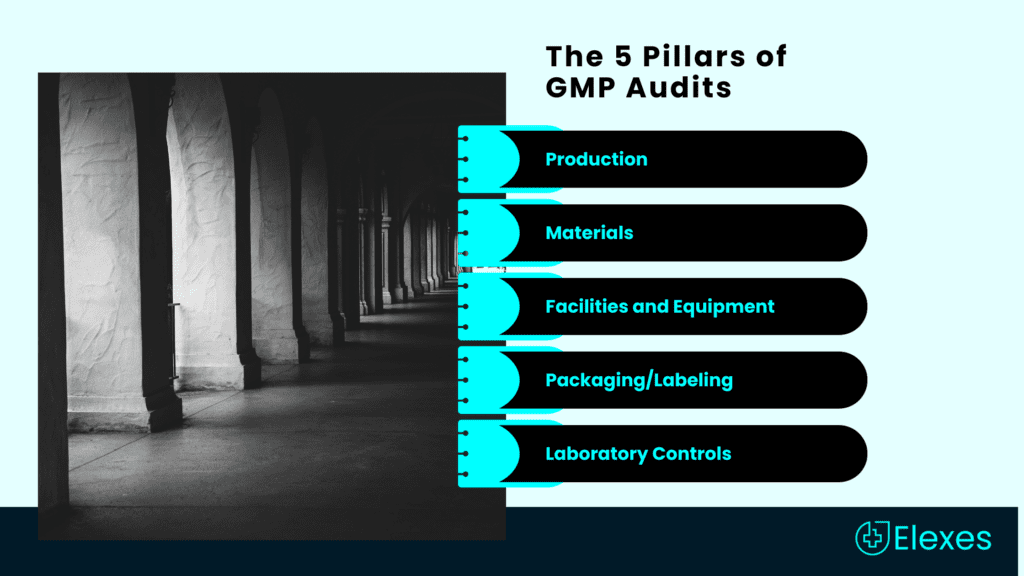
To comprehend the multifaceted nature of GMP audits, envision them as an intricate puzzle composed of five essential pieces, each representing a vital aspect of manufacturing:
- Production: This piece involves the core manufacturing processes, ensuring they adhere to defined protocols, resulting in products that meet quality and safety standards.
- Materials: GMP audits delve into the procurement and use of materials, verifying that they meet stringent quality criteria and are sourced from reputable suppliers.
- Facilities and Equipment: Auditors meticulously inspect the manufacturing facility and equipment, ensuring they are maintained, sanitized, and calibrated to deliver consistent quality.
- Packaging/Labeling: Every detail matters, down to the packaging and labeling as well. Auditors confirm that these elements are precise, accurate, and compliant with regulations.
- Laboratory Controls: In industries where rigorous testing is imperative, GMP audits extend their scrutiny to laboratory practices, ensuring that testing procedures are reliable and yield accurate results.
In essence, GMP audits are the compass that guides manufacturers, ensuring they stay on the path of compliance, consistency, and customer satisfaction.
Whether conducted by private entities or government bodies, these audits are a cornerstone of good business practices, shedding light on areas that need refinement and reinforcing the commitment to excellence.
Inside the GMP Audit Process
The GMP audit process unfolds through distinct phases:
- Audit Planning: The auditor strategizes the audit, outlining areas for scrutiny.
- Opening Meeting: A formal introduction and alignment of objectives.
- Fact Collection: Meticulous review of documents, interviews, and observations.
- Documentation: Documenting findings and making a comprehensive audit report.
Phase Breakdown:
- Phase 1 – Audit Planning: Collect initial information through interviews and record examination.
- Phase 2 – Observation and Assessment: Deep dive into processes, documentation, and batch data, resulting in an audit report.
This structured process ensures compliance, quality, and regulatory adherence, fostering continuous improvement.
How to Be Prepared for GMP Audits: A Strategic Approach
Preparing for the scrutiny of a GMP audit is essential which is why it is important to equip yourself with a well-constructed toolkit—each tool serves a purpose in ensuring a seamless and successful audit process.
So, how can manufacturing companies gear up for internal or external audits?
Let’s dive into a strategic approach, drawing insights from industry best practices:
Learn from the Past
SOPs and Agenda Development
Team Preparation
Document Accessibility
GMP Compliance Checklist
Familiarize Your Team
Pro Tips for a Smooth GMP Audit
Hosting a GMP audit can be a nerve-wracking experience, but with some strategic planning and attention to detail, you can smoothly get through the audit. Here are some pro tips to help you ace your next GMP audit:

Documentation Mastery
Ensure your company’s official documents are meticulously organized and up-to-date. Having all your documentation in order is paramount.
Make necessary changes or additions in advance to prevent any last-minute surprises during the audit. Remember, a well-documented ship sails smoothly through audit storms.
Availability and Comfort
During the audit process, it’s crucial to have someone available at all times. Additionally, ensure that you have comfortable facilities in place where both audit teams can work without discomfort.
This minimizes stress, fosters a conducive working environment, and prevents potential miscommunication issues down the road.
The War Room Essentials
Your “war room” should be well-prepared. Stock it with vital resources, including an organizational chart, facility layout, Quality System Manual, Standard Operating Procedures (SOPs), and Staff Training Documentation.
Having these tools readily accessible ensures that you’re well-equipped to address auditors’ questions and requirements promptly.
Common Pitfalls to Avoid
While striving for a successful GMP audit, it’s equally important to steer clear of common pitfalls that could jeopardize the process. Here are some mistakes to avoid:
Unpreparedness
One big no-no in getting ready for an audit is not being ready. Ensure that your team is well-versed in GMP regulations and understands the audit process thoroughly. Lack of preparedness can lead to setbacks.
Documentation Disarray
Not having all your paperwork in order can really mess up the whole audit. Ensure that all required documents are up-to-date, readily accessible, and aligned with regulatory requirements.
Wrong Personnel
Having the wrong individuals present during the audit can hinder the process. Ensure that the team members attending the audit possess the necessary knowledge and expertise to address auditors’ inquiries effectively.
Equipment Shortfalls
Imagine not being able to test a product during the audit because you lack the necessary testing equipment. To prevent this, ensure that you have the right equipment on hand, calibrated, and ready for use.
Best Practices for GMP Auditing Excellence
GMP auditing is not just about compliance; it’s an opportunity to showcase your commitment to quality and safety. Here are some best practices to elevate your GMP audit experience:
Staff Awareness
Keep your staff well-informed about the upcoming audit.
Training and Preparation
Invest in your staff’s training and preparation for the audit. Equipped with knowledge and readiness, they become valuable assets during the audit.
Team Readiness
Schedule a team meeting before the assessment or inspection to align everyone’s expectations and preparations. Knowing what’s expected of them during the audit helps team members navigate unforeseen challenges with confidence.
Hire Professional Regulatory Consultant
It is essential for manufacturing companies to hire professional regulatory consultants to ensure that you are all ready for the GMP audits and are in compliance with all the regulations. Elexes Medical Consulting is one of the trusted consultants across the globe who can assist you with GMP Audits and more…
In the intricate world of GMP audits, being prepared, avoiding common pitfalls, and embracing best practices can transform a potentially daunting process into an opportunity for growth and refinement.
Final Words
With these tips in your arsenal, you’ll not only survive but thrive in the world of GMP audits, reinforcing your organization’s commitment to excellence and compliance.
In conclusion, GMP audits are a fundamental aspect of ensuring product safety and quality in regulated industries such as pharmaceuticals, medical devices, and food.
By understanding their purpose and adequately preparing for them, companies can navigate these audits successfully, maintain compliance with regulations, and consistently produce high-quality products.
Remember that compliance is an ongoing commitment, and continuous improvement is key to long-term success in GMP audits.
Want to know more about GMP audits? Simply contact us.





















