What do the applicants of 510(k) or other regulatory applications or companies looking to register or import need to pay?
The FDA has amended the user fee for the fiscal year 2022, and there is about a 2.5% rise in the fees for various medical device manufacturers, importers and distributors on the regulatory applications where they need FDA’s review, or for registration that is to be done with the FDA. The amendment is effective until September 30, 2022, and is a less steep increase than the 7 % increase for 2021.
Below are the amended User Fees of FY 2022 for market applications, notices or registrations:
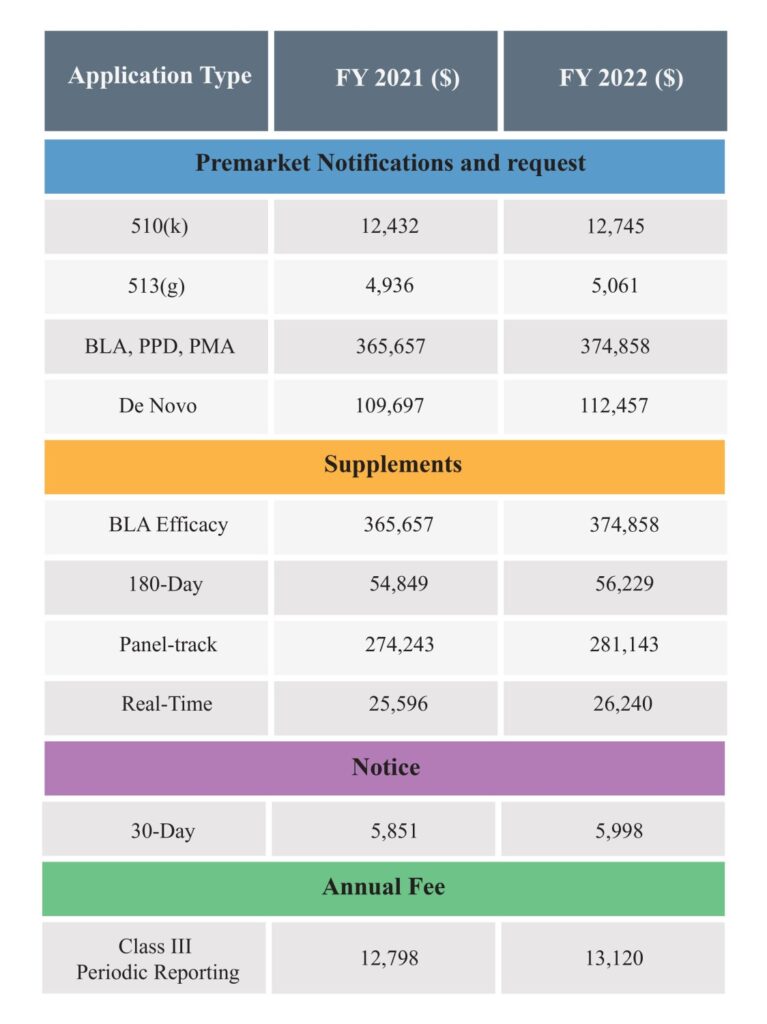
It is important to know that a Medical Device Manufacturer, Distributor or Importer and others (who want to submit or renew applications or register with the FDA) can benefit from fee reductions and waivers for certain types of market submission through the FDA’s Small Business Determination Requests program.
The following are the application types for which a medical device company can pay reduced fees.
- 510(k) submission
- De Novo request
- BLA, PPD, PMA
- PMR
- Premarket Application Supplements and Annual Reports
- 513(g)
Who can qualify as a small business?
Want to know if you qualify for the reduced small business fees? Here’s some info to help:
If the turnover of your business (including sales or receipts from your affiliates) is less than $100 million, the Center for Devices and Radiological Health (CDRH) can certify your business as a small business. There are no fees associated with the submission to get this designation. However, you must be able to produce the necessary evidence needed to back up the numbers. The application for a small business can be done by following some defined steps as outlined here – Small business qualification steps by Elexes.

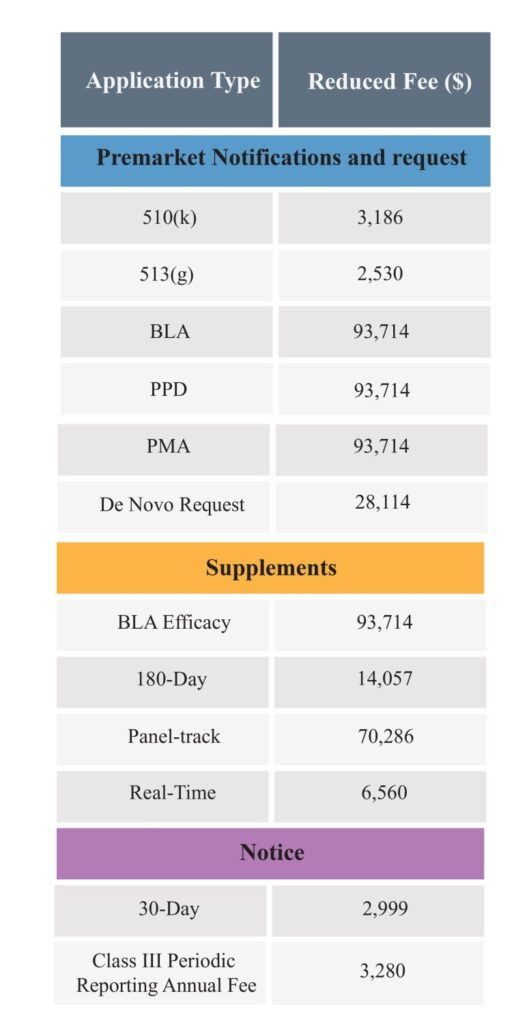
The reduced fees for the small business is as follows:
In the world of medical device regulation and quality, change is the only constant, and to continue selling within the US or internationally it is critical to ensure that you are always aware of the updates and are staying compliant. Our FTEs take pride in keeping up with latest modifications and helping medical device companies in better budget planning or planning for regulatory expenses, especially when it comes to the payable amounts to the FDA or other regulatory bodies. We are just an email away, please feel free to reach out to jennifer@elexes.com.
#elexes #iso13485 #healthcare #fda #us #fee #510k #denovo #mdr #cer #medicaldevice




















