What is Software as a Medical Device (SaMD)?
The International Medical Device Regulators Forum (IMDRF) defines Software as a Medical Device (SaMD) as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.”
Notes:-
- SaMD is a medical device and includes in-vitro diagnostic (IVD) medical device.
- SaMD is capable of running on general purpose (non-medical purpose) computing platforms
- “without being part of” means software not necessary for a hardware medical device to achieve its intended medical purpose;
- Software does not meet the definition of SaMD if its intended purpose is to drive a hardware medical device.
- SaMD may be used in combination (e.g., as a module) with other products including medical devices;
- SaMD may be interfaced with other medical devices, including hardware medical devices and other SaMD software, as well as general purpose software
- Mobile apps that meet the definition above are considered SaMD.
What is and is not Software as a Medical Device?
Here are the software as a medical device examples, and the non software as a medical device examples:
| SaMD | Non SaMD |
|---|---|
| Mobile Health Apps: Applications that track vital signs, monitor chronic conditions (like diabetes or hypertension), provide medication reminders, or offer personalized health advice fall into this category. | Electronic Health Records (EHR) Systems: While EHR systems store and manage patient health records electronically, they are often not considered SaMD as they primarily serve as tools for healthcare providers to maintain records rather than directly diagnose, treat, or monitor patients. |
| Diagnostic Software: Software that aids in diagnosing medical conditions, such as image analysis software that helps radiologists identify and interpret medical images like X-rays, MRIs, and CT scans. | Hospital Management Software: Software used for administrative purposes within healthcare facilities, such as scheduling appointments, managing billing and payments, and maintaining inventory. |
| Clinical Decision Support Systems: Healthcare providers receive data-driven treatment recommendations based on patient data and clinical guidelines. | Practice Management Software: Similar to hospital management software, these applications are used by private practices to manage appointments, patient records, billing, and other administrative tasks. |
| Telemedicine Platforms: Software platforms that enable remote patient-doctor consultations, allowing healthcare providers to diagnose and treat patients virtually. | Medical Billing and Coding Software: These tools help healthcare providers and medical billing professionals generate accurate medical bills and apply appropriate codes for insurance reimbursement. |
| Electronic Health Records (EHR) Systems: While not exclusively SaMD, these systems involve software used to manage and store patient health records electronically. | Medical Education and Training Software: Software designed to educate medical students, residents, and healthcare professionals about medical procedures, anatomy, and treatment protocols. |
| Remote Monitoring Systems: Software that enables remote monitoring of patients’ vital signs and health data, often used for patients with chronic conditions. | Medical Research Software: Applications used in research settings to analyze medical data, perform statistical analysis, and draw conclusions from medical studies. |
| Radiology Software: Software that assists in processing, analyzing, and visualizing medical images from various imaging modalities. | Healthcare Analytics Software: Software that processes and analyzes large volumes of healthcare data to extract insights and trends for decision-making purposes. |
| Smart Wearable Devices: Wearable devices that monitor various health metrics like heart rate, sleep patterns, physical activity, and more, and provide insights for users and healthcare professionals. | Health Information Exchange (HIE) Platforms: These platforms facilitate the sharing of electronic health information among different healthcare providers and organizations to improve patient care coordination. |
| Medical Imaging Software: Software used for advanced medical imaging tasks, like 3D reconstruction, visualization, and analysis of medical images. | Medical Device Calibration and Maintenance Software: Software used by technicians to calibrate, maintain, and manage the performance of medical equipment. |
| Therapeutic Software: Some software applications are used directly for medical treatment, such as neurofeedback software for treating attention disorders. | Laboratory Information Management Systems (LIMS): Software used in laboratories to manage sample tracking, testing processes, and data recording. |
| Health Monitoring Software for In-Vitro Diagnostics: Software used to interpret results from diagnostic tests like blood tests, genetic tests, and other laboratory analyses. | Patient Portals: Digital ecosystems designed to provide patients with the ability to retrieve their medical records, arrange appointments, engage in direct communication with healthcare professionals, and peruse their test results seamlessly through online interfaces. |
| Pacemaker and Implantable Device Programming: Software used by healthcare professionals to program and adjust settings of implantable medical devices like pacemakers and defibrillators. | Health and Wellness Apps: Apps focused on general health and wellness, including fitness tracking, meditation, sleep monitoring, and nutrition tracking. It is important to note that these apps are not intended to make medical diagnoses or provide medical treatments. |
| Fertility Tracking Apps: Apps that help individuals track menstrual cycles, ovulation, and fertility windows to aid in family planning. | Appointment Scheduling Software: Software tools used by healthcare providers and medical facilities to manage patient appointments and schedules. |
| Rehabilitation and Physical Therapy Software: Software applications that guide patients through exercises and rehabilitation protocols. | Healthcare Communication Platforms: Applications that facilitate communication among healthcare professionals, patients, and caregivers, often through secure messaging and video conferencing. |
| Personalized Treatment Planning Software: Software that takes patient data into account to help healthcare providers create tailored treatment plans. | Medical Data Visualization Tools: Software that helps healthcare professionals visualize complex medical data, such as trends in patient outcomes or population health. |
SaMD Global Regulations Overview
Global regulations for SaMD have been evolving to ensure patient safety and product effectiveness. Organizations such as the FDA in the United States, the European Medicines Agency (EMA) in the EU, and various global regulatory entities have been actively formulating guidelines to categorize Software as a Medical Device (SaMD), evaluate its risk profile, and define suitable regulatory pathways. These regulations aim to strike a balance between innovation and patient safety, requiring medical device manufacturing companies to demonstrate software quality, clinical validity, and ongoing post-market surveillance. The regulatory landscape continues to evolve as technology advances, emphasizing the importance of staying updated with the latest guidelines to ensure compliance and the safe deployment of SaMD globally.
SaMD classifications as per IMDRF
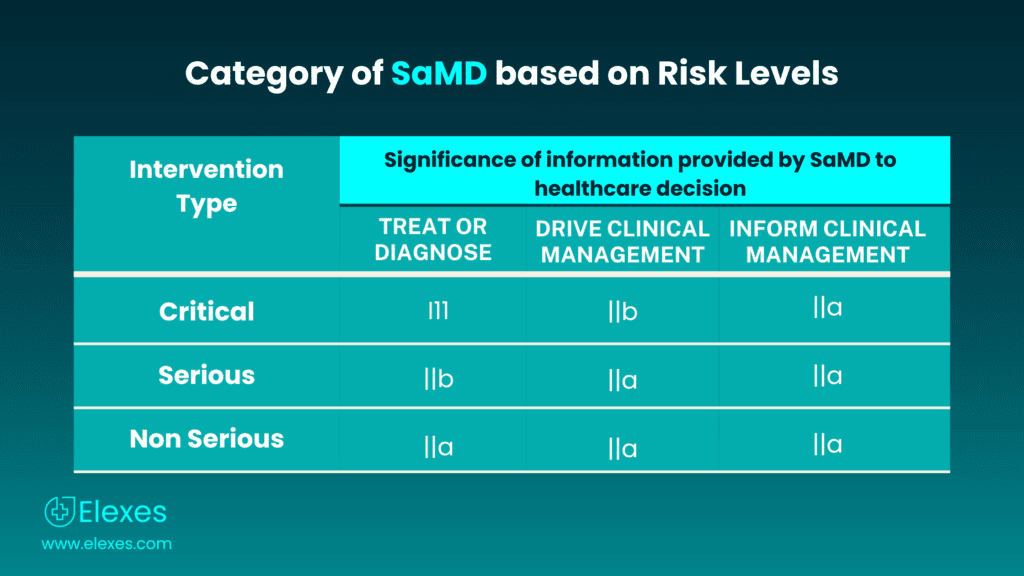
The International Medical Device Regulators Forum (IMDRF) offers direction on the classification of Software as a Medical Device (SaMD), taking into account the inherent risks associated with the software application. The IMDRF SaMD categorization framework consists of four categories, which are defined as follows:
| Intervention type | Treat or diagnose | Drive clinical management | Informs clinical management |
|---|---|---|---|
| Critical | III | IIb | IIa |
| Serious | IIb | IIa | IIa |
| Non-serious | IIa | IIa | IIa |
⦿ SaMD Category I: This category includes low-risk software that is unlikely to cause harm to the patient or user. It typically involves software used for administrative purposes, such as patient management, appointment scheduling, or maintaining electronic health records.
⦿ SaMD Category II: Category II includes software with a moderate level of risk. This category encompasses software that is used for diagnostic or therapeutic purposes but does not have a direct impact on patient safety.
For example, software that aids healthcare professionals in making clinical decisions or software used for tracking vital signs may fall into this category.
⦿ SaMD Category III: Category III consists of high-risk software that directly impacts patient safety. This category includes software that is used for critical diagnostic or therapeutic purposes and has the potential to cause harm if it malfunctions.
Medical imaging software, software-controlled devices used for treatment, and software that monitors or manages life-supporting equipment are examples of Category III SaMD.
⦿ SaMD Category IV: This is the highest-risk category and includes software that is implanted within the human body or is directly responsible for sustaining or saving human life.
Category IV SaMD includes software used in implantable medical devices, such as pacemakers or insulin pumps.
SaMD Classifications in US FDA
As per the latest FDA guidance, Content of Premarket Submissions for Device Software Functions, June 2023, the three levels of concern have been supplanted with two levels of documentation:
- Enhanced Documentation – In cases where the failure or malfunction of any device software function(s) may lead to a critical situation with a potential risk of severe injury or fatality, either to a patient, the device user, or individuals in the usage environment.
- Basic Documentation – Pertaining to scenarios where the failure or flaw of any device software function(s) is less likely to result in a critical situation with a potential risk of severe injury or death, either to a patient, the device user, or others in the usage environment.
SaMD classifications in EU
The European Commission’s Medical Device Coordination Group (MDCG) categorizes Medical Device Software (MDSW) into Class I, II, or III. However, for devices falling under the In Vitro Diagnostic Regulation (IVDR), the Medical Device Regulation (MDR) defines four classes with the labels A, B, C, and D.
The IEC 62304
IEC 62304 stands as a globally recognized standard, offering a structured framework for the software life cycle processes inherent to medical device software. Issued by the International Electrotechnical Commission (IEC) under the title “Medical device software – Software life cycle processes,” this standard holds significance in guiding the development of software intricately woven into medical devices, encompassing applications responsible for overseeing or regulating the device’s functionality.
Here’s an overview of the key aspects and requirements of IEC 62304:
- Scope: IEC 62304 is applicable to the software used in medical devices, including embedded software, stand-alone software, and software that is part of a larger system.
- Software Classification: The standard classifies software into three categories based on the potential risks associated with its use: Class A (low risk), Class B (medium risk), and Class C (high risk).
- Software Life Cycle Processes: IEC 62304 defines various software life cycle processes that should be followed during the development of medical device software. These processes include:
- Software development.
- Software maintenance.
- Software configuration management.
- Software risk management.
- Software problem resolution.
- Software verification and validation.
- Requirements for Each Life Cycle Process: The standard outlines specific requirements for each of these processes, including documentation, planning, and testing requirements.Throughout the software development life cycle, traceability, risk management, and quality assurance are crucial.
- Software Development Lifecycle Phases: IEC 62304 divides the software development life cycle into various phases, such as:
- Requirements analysis and specification.
- Architectural design.
- Detailed design and coding.
- Testing (including unit testing, integration testing, and system testing).
- Software release.
- Software Risk Management: The standard requires a comprehensive software risk management process, including the identification, analysis, and mitigation of potential risks associated with the software. Risk management is crucial, especially for software in Class B and Class C devices.
- Documentation and Traceability: IEC 62304 mandates thorough documentation of all phases of the software life cycle. This includes documenting design decisions, test results, and risk assessments. Traceability ensures that each requirement is linked to the corresponding design, code, and test components.
- Validation and Verification: The standard emphasizes the importance of software validation (confirming that the software meets user needs and intended uses) and verification (ensuring that the software conforms to its specifications) through testing and other techniques.
- Maintenance and Configuration Management: IEC 62304 includes guidelines for software maintenance activities, including handling software changes, problem resolution, and version control.
- Compliance and Certification: Compliance with IEC 62304 is often a prerequisite for regulatory approval and certification of medical devices, especially in countries like the United States (FDA) and the European Union (CE marking).
References
- International Medical Device Regulators Forum – SaMD
- Software as a Medical Device (SaMD) – USFDA
- Ultimate Guide to Software as a Medical Device (SaMD)
- What are the Regulatory Expectations for Software as a Medical Device (SaMD)?
- Software as a medical device (SAMD) – classification overview By Wendy Levine
- New FDA Guidance on Software Documentation for Medical Devices By Sonali Hinge





















